Separation and Purification Technology ( IF 8.1 ) Pub Date : 2022-03-10 , DOI: 10.1016/j.seppur.2022.120810 Chen-Yan Hu , Ye-Ye Zhu , Bin Xu , Tian-Yang Zhang , Yi-Li Lin , Cun Xiong , Qiang-Bing Wang , Dan-Dan Huang , Ling Xu
|
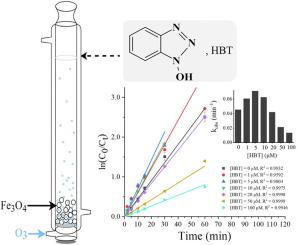
This study explored the degradation of an iodine X-ray contrast media, iohexol, under heterogeneous ozone-catalyzed oxidation by adding Fe3O4 and 1-hydroxybenzotriazole (HBT). Results show that increasing the dosages of Fe3O4 catalyst and ozone as well as solution pH were beneficial to the degradation of iohexol. The presence of low background humic acid (HA) concentration (≤ 10 mg/L) promoted iohexol degradation, but high HA concentration (≥ 20 mg/L) inhibited it. Adding HBT exhibited a similar effect on iohexol degradation as HA. The degradation rate constant of iohexol was calculated as 0.07108 min-1 in the Fe3O4/O3/HBT process ([HBT] = 5 μM) at pH 7.0, which was approximately 1.5 times higher than that in the Fe3O4/O3 process (0.04533 min-1), and 4 times higher than that in the O3 process (0.01742 min-1). Fe3O4/O3/HBT process was pH-dependent with high degradation efficiency of iohexol at pH 5 or 7. Singlet oxygen (1O2), hydroxyl radical (), and superoxide radical () have been identified in the Fe3O4/O3/HBT process, and the conversion of Fe2+ to Fe3+ was more obvious on the surface of Fe3O4 compared to that in the Fe3O4/O3 process. Transformation intermediates were identified, and the degradation pathways of iohexol were proposed. Overall, Fe3O4/O3 could enhance iohexol degradation compared with ozone-only system, and adding trace HBT in Fe3O4/O3 system can further enhance heterogeneous ozone-catalyzed process for rapid organic matter degradation.
中文翻译:

1-羟基苯并三唑存在下Fe3O4催化臭氧降解碘海醇的性能、转化机理和途径
本研究通过添加 Fe 3 O 4和 1-羟基苯并三唑 (HBT)探索了碘 X 射线造影剂碘海醇在非均相臭氧催化氧化下的降解。结果表明,增加Fe 3 O 4催化剂和臭氧的用量以及溶液pH值有利于碘海醇的降解。低背景腐植酸 (HA) 浓度 (≤ 10 mg/L) 的存在促进了碘海醇的降解,但高 HA 浓度 (≥ 20 mg/L) 抑制了它。添加 HBT 对碘海醇降解的影响与 HA 相似。在Fe 3 O 4 /O 3中,碘海醇的降解速率常数计算为0.07108 min -1/HBT 工艺 ([HBT] = 5 μM) 在 pH 7.0 时,约为 Fe 3 O 4 /O 3工艺 (0.04533 min -1 )的 1.5 倍,是O 3的 4 倍过程(0.01742 分钟-1)。Fe 3 O 4 /O 3 /HBT 过程具有 pH 依赖性,在 pH 5 或 7 时碘海醇的降解效率高。单线态氧 ( 1 O 2 )、羟基自由基 () 和超氧自由基 ()在Fe 3 O 4 /O 3 /HBT工艺中发现,与Fe 3 O 4 /O相比, Fe 3 O 4表面Fe 2+向Fe 3+的转化更为明显3过程。鉴定了转化中间体,并提出了碘海醇的降解途径。总体而言,与纯臭氧系统相比,Fe 3 O 4 /O 3可以增强碘海醇降解,并且在 Fe 3 O 4 /O 3中添加微量 HBT系统可以进一步增强非均相臭氧催化过程的快速有机物降解。












































 京公网安备 11010802027423号
京公网安备 11010802027423号