当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective [1,2]-Stevens rearrangement of thiosulfonates to construct dithio-substituted quaternary carbon centers
Chemical Science ( IF 7.6 ) Pub Date : 2022-03-11 , DOI: 10.1039/d2sc00419d Linfeng Hu 1 , Jinzhao Li 1 , Yongyan Zhang 1 , Xiaoming Feng 1 , Xiaohua Liu 1
Chemical Science ( IF 7.6 ) Pub Date : 2022-03-11 , DOI: 10.1039/d2sc00419d Linfeng Hu 1 , Jinzhao Li 1 , Yongyan Zhang 1 , Xiaoming Feng 1 , Xiaohua Liu 1
Affiliation
|
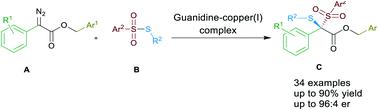
An enantioselective [1,2] Stevens rearrangement was realized by using chiral guanidine and copper(I) complexes. Bis-sulfuration of α-diazocarbonyl compounds was developed through using thiosulfonates as the sulfenylating agent. It was undoubtedly an atom-economic process providing an efficient route to access novel chiral dithioketal derivatives, affording the corresponding products in good yields (up to 90% yield) and enantioselectivities (up to 96 : 4 er). A novel catalytic cycle was proposed to rationalize the reaction process and enantiocontrol.
中文翻译:

硫代磺酸盐的对映选择性 [1,2]-Stevens 重排构建二硫代取代的季碳中心
使用手性胍和铜 ( I ) 配合物实现了对映选择性 [1,2] Stevens 重排。通过使用硫代磺酸盐作为亚磺酰化剂,开发了 α-重氮羰基化合物的双硫化。毫无疑问,这是一种原子经济过程,为获得新型手性二硫缩酮衍生物提供了有效途径,以良好的产率(高达 90% 的产率)和对映选择性(高达 96 : 4 er)提供相应的产品。提出了一种新的催化循环来合理化反应过程和对映体控制。
更新日期:2022-03-11
中文翻译:

硫代磺酸盐的对映选择性 [1,2]-Stevens 重排构建二硫代取代的季碳中心
使用手性胍和铜 ( I ) 配合物实现了对映选择性 [1,2] Stevens 重排。通过使用硫代磺酸盐作为亚磺酰化剂,开发了 α-重氮羰基化合物的双硫化。毫无疑问,这是一种原子经济过程,为获得新型手性二硫缩酮衍生物提供了有效途径,以良好的产率(高达 90% 的产率)和对映选择性(高达 96 : 4 er)提供相应的产品。提出了一种新的催化循环来合理化反应过程和对映体控制。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号