当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Radical S-Adenosyl-l-methionine Adenosylation: Radical Intermediates and the Catalytic Competence of the 5′-Deoxyadenosyl Radical
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-03-08 , DOI: 10.1021/jacs.1c13706 Maike N Lundahl 1 , Raymond Sarksian 2 , Hao Yang 3 , Richard J Jodts 3 , Adrien Pagnier 1 , Donald F Smith 1 , Martín A Mosquera 1 , Wilfred A van der Donk 2 , Brian M Hoffman 3 , William E Broderick 1 , Joan B Broderick 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-03-08 , DOI: 10.1021/jacs.1c13706 Maike N Lundahl 1 , Raymond Sarksian 2 , Hao Yang 3 , Richard J Jodts 3 , Adrien Pagnier 1 , Donald F Smith 1 , Martín A Mosquera 1 , Wilfred A van der Donk 2 , Brian M Hoffman 3 , William E Broderick 1 , Joan B Broderick 1
Affiliation
|
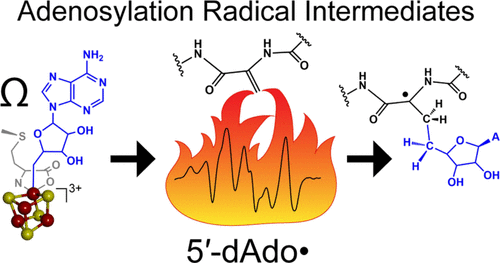
Radical S-adenosyl-l-methionine (SAM) enzymes employ a [4Fe–4S] cluster and SAM to initiate diverse radical reactions via either H-atom abstraction or substrate adenosylation. Here we use freeze-quench techniques together with electron paramagnetic resonance (EPR) spectroscopy to provide snapshots of the reaction pathway in an adenosylation reaction catalyzed by the radical SAM enzyme pyruvate formate-lyase activating enzyme on a peptide substrate containing a dehydroalanine residue in place of the target glycine. The reaction proceeds via the initial formation of the organometallic intermediate Ω, as evidenced by the characteristic EPR signal with g∥ = 2.035 and g⊥ = 2.004 observed when the reaction is freeze-quenched at 500 ms. Thermal annealing of frozen Ω converts it into a second paramagnetic species centered at giso = 2.004; this second species was generated directly using freeze-quench at intermediate times (∼8 s) and unequivocally identified via isotopic labeling and EPR spectroscopy as the tertiary peptide radical resulting from adenosylation of the peptide substrate. An additional paramagnetic species observed in samples quenched at intermediate times was revealed through thermal annealing while frozen and spectral subtraction as the SAM-derived 5′-deoxyadenosyl radical (5′-dAdo•). The time course of the 5′-dAdo• and tertiary peptide radical EPR signals reveals that the former generates the latter. These results thus support a mechanism in which Ω liberates 5′-dAdo• by Fe–C5′ bond homolysis, and the 5′-dAdo• attacks the dehydroalanine residue of the peptide substrate to form the adenosylated peptide radical species. The results thus provide a picture of a catalytically competent 5′-dAdo• intermediate trapped just prior to reaction with the substrate.
中文翻译:

自由基 S-腺苷-l-甲硫氨酸腺苷化的机制:自由基中间体和 5'-脱氧腺苷自由基的催化能力
自由基S -腺苷-l-甲硫氨酸(SAM) 酶使用 [4Fe–4S] 簇和 SAM 通过 H 原子提取或底物腺苷化启动多种自由基反应。在这里,我们使用冷冻淬火技术和电子顺磁共振 (EPR) 光谱学来提供由自由基 SAM 酶丙酮酸甲酸裂解酶激活酶在含有脱氢丙氨酸残基的肽底物上催化的腺苷化反应中的反应途径快照目标甘氨酸。反应通过有机金属中间体 Ω 的初始形成进行, g ∥ = 2.035 和g ⊥的特征 EPR 信号证明了这一点= 2.004 当反应在 500 毫秒时被冻结淬灭时观察到。冷冻 Ω 的热退火将其转化为以g iso为中心的第二种顺磁性物质= 2.004; 这第二个物种是在中间时间(~8 s)使用冷冻淬火直接产生的,并通过同位素标记和 EPR 光谱明确鉴定为肽底物腺苷化产生的三级肽自由基。在中间时间淬火的样品中观察到的另一种顺磁性物质通过冷冻时的热退火和光谱减法揭示为 SAM 衍生的 5'-脱氧腺苷自由基 (5'-dAdo•)。5'-dAdo• 和三级肽自由基 EPR 信号的时间进程表明前者产生后者。因此,这些结果支持一种机制,其中 Ω 通过 Fe–C5' 键均裂释放 5'-dAdo•,并且 5'-dAdo• 攻击肽底物的脱氢丙氨酸残基以形成腺苷化肽自由基种类。
更新日期:2022-03-08
中文翻译:

自由基 S-腺苷-l-甲硫氨酸腺苷化的机制:自由基中间体和 5'-脱氧腺苷自由基的催化能力
自由基S -腺苷-l-甲硫氨酸(SAM) 酶使用 [4Fe–4S] 簇和 SAM 通过 H 原子提取或底物腺苷化启动多种自由基反应。在这里,我们使用冷冻淬火技术和电子顺磁共振 (EPR) 光谱学来提供由自由基 SAM 酶丙酮酸甲酸裂解酶激活酶在含有脱氢丙氨酸残基的肽底物上催化的腺苷化反应中的反应途径快照目标甘氨酸。反应通过有机金属中间体 Ω 的初始形成进行, g ∥ = 2.035 和g ⊥的特征 EPR 信号证明了这一点= 2.004 当反应在 500 毫秒时被冻结淬灭时观察到。冷冻 Ω 的热退火将其转化为以g iso为中心的第二种顺磁性物质= 2.004; 这第二个物种是在中间时间(~8 s)使用冷冻淬火直接产生的,并通过同位素标记和 EPR 光谱明确鉴定为肽底物腺苷化产生的三级肽自由基。在中间时间淬火的样品中观察到的另一种顺磁性物质通过冷冻时的热退火和光谱减法揭示为 SAM 衍生的 5'-脱氧腺苷自由基 (5'-dAdo•)。5'-dAdo• 和三级肽自由基 EPR 信号的时间进程表明前者产生后者。因此,这些结果支持一种机制,其中 Ω 通过 Fe–C5' 键均裂释放 5'-dAdo•,并且 5'-dAdo• 攻击肽底物的脱氢丙氨酸残基以形成腺苷化肽自由基种类。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号