当前位置:
X-MOL 学术
›
ACS Mater. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Reconstruction on Uniform Cu Nanodisks Boosted Electrochemical Nitrate Reduction to Ammonia
ACS Materials Letters ( IF 9.6 ) Pub Date : 2022-03-08 , DOI: 10.1021/acsmaterialslett.2c00149 Keming Wu 1 , Congcong Sun 1 , Zhenni Wang 1 , Qian Song 1 , Xiaoxia Bai 1 , Xin Yu 2 , Qiang Li 1 , Zheng Wang 1, 3 , Hui Zhang 1 , Jian Zhang 4 , Xin Tong 5 , Yanping Liang 1 , Ajit Khosla 1 , Zhenhuan Zhao 1
ACS Materials Letters ( IF 9.6 ) Pub Date : 2022-03-08 , DOI: 10.1021/acsmaterialslett.2c00149 Keming Wu 1 , Congcong Sun 1 , Zhenni Wang 1 , Qian Song 1 , Xiaoxia Bai 1 , Xin Yu 2 , Qiang Li 1 , Zheng Wang 1, 3 , Hui Zhang 1 , Jian Zhang 4 , Xin Tong 5 , Yanping Liang 1 , Ajit Khosla 1 , Zhenhuan Zhao 1
Affiliation
|
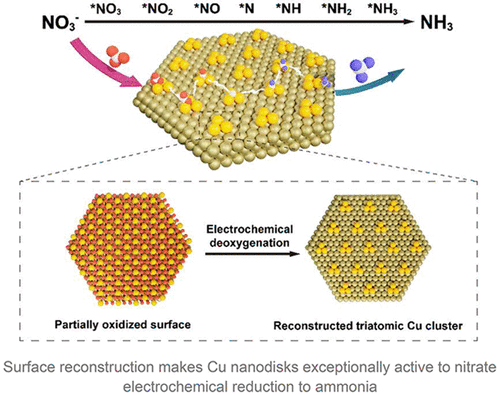
The Haber–Bosch (HB) process has provided most of commercial ammonia at the expense of high energy consumption and high CO2 emission. Nitrate electroreduction is showing great potential as an alternative route for the green and scale-up synthesis of ammonia at ambient conditions. However, the performance has lagged due to lack of efficient electrocatalysts. In this work, we present the facile synthesis of uniform Cu nanodisks with exposed (111) facets as highly active electrocatalyst for electrochemical ammonia synthesis, delivering a high ammonia yield of 2.16 mg mg–1cat h–1 and a maximum Faradaic efficiency of 81.1% at −0.5 V versus a reversible hydrogen electrode (RHE). The remarkable activity is originated from the surface reconstructed triatomic Cu clusters due to the cathodic deoxygenation process. As a result, the reconstructed surface shows enhanced affinity to the adsorption of nitrate ions which undergo successive break of three N–O bonds, followed by subsequent formation of three N–H bonds to finally form NH3. The present study provides the feasible preparation of Cu based advanced catalysts and a unique insight into the mechanism of nitrate electroreduction.
中文翻译:

均匀铜纳米盘的表面重建促进硝酸盐电化学还原为氨
Haber-Bosch (HB) 工艺以高能耗和高 CO 2排放为代价提供了大部分商业氨。硝酸盐电还原作为在环境条件下绿色和放大合成氨的替代途径显示出巨大的潜力。然而,由于缺乏有效的电催化剂,性能已经落后。在这项工作中,我们展示了具有暴露 (111) 晶面的均匀铜纳米盘的简便合成方法,作为电化学氨合成的高活性电催化剂,氨产量高达 2.16 mg mg –1 cat h –1与可逆氢电极 (RHE) 相比,在 -0.5 V 时的最大法拉第效率为 81.1%。由于阴极脱氧过程,显着的活性源于表面重建的三原子铜簇。结果,重建的表面对硝酸根离子的吸附具有增强的亲和力,硝酸根离子经历三个N-O键的连续断裂,随后形成三个N-H键,最终形成NH 3。本研究为Cu基先进催化剂的制备提供了可行的方法,并对硝酸盐电还原机理提供了独特的见解。
更新日期:2022-03-08
中文翻译:

均匀铜纳米盘的表面重建促进硝酸盐电化学还原为氨
Haber-Bosch (HB) 工艺以高能耗和高 CO 2排放为代价提供了大部分商业氨。硝酸盐电还原作为在环境条件下绿色和放大合成氨的替代途径显示出巨大的潜力。然而,由于缺乏有效的电催化剂,性能已经落后。在这项工作中,我们展示了具有暴露 (111) 晶面的均匀铜纳米盘的简便合成方法,作为电化学氨合成的高活性电催化剂,氨产量高达 2.16 mg mg –1 cat h –1与可逆氢电极 (RHE) 相比,在 -0.5 V 时的最大法拉第效率为 81.1%。由于阴极脱氧过程,显着的活性源于表面重建的三原子铜簇。结果,重建的表面对硝酸根离子的吸附具有增强的亲和力,硝酸根离子经历三个N-O键的连续断裂,随后形成三个N-H键,最终形成NH 3。本研究为Cu基先进催化剂的制备提供了可行的方法,并对硝酸盐电还原机理提供了独特的见解。































 京公网安备 11010802027423号
京公网安备 11010802027423号