当前位置:
X-MOL 学术
›
Energy Environ. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unveiling the Underlying Mechanism of Transition Metal Atoms Anchored Square Tetracyanoquinodimethane Monolayers as Electrocatalysts for N2 Fixation,揭示过渡金属原子锚定方形四氰基醌二甲烷单分子层作为 N2 固定电催化剂的潜在机制
Energy & Environmental Materials ( IF 13.0 ) Pub Date : 2021-09-16 , DOI: 10.1002/eem2.12277
Sheng‐Yao Lv 1, 2 , Chun‐Xiang Huang 1, 2 , Guoliang Li 2 , Li‐Ming Yang 1
Energy & Environmental Materials ( IF 13.0 ) Pub Date : 2021-09-16 , DOI: 10.1002/eem2.12277
Sheng‐Yao Lv 1, 2 , Chun‐Xiang Huang 1, 2 , Guoliang Li 2 , Li‐Ming Yang 1
Affiliation
|
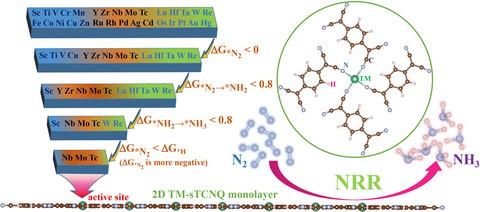
We for the first time systematically studied the structures and electrochemical nitrogen reduction reaction properties of two-dimensional single transition-metal anchored square tetracyanoquinodimethane monolayers (labeled as: TM-sTCNQ, TM = 3d, 4d, 5d series transition metals) by employing density functional theory method. Through high-throughput screenings and full reaction path researches, two promising electrochemical nitrogen reduction reaction catalysts Nb-sTCNQ and Mo-sTCNQ have been obtained. The nitrogen reduction reaction onset potential on Nb-sTCNQ is as low as −0.48 V. Furthermore, the Nb-sTCNQ catalyst can quickly desorb NH3 produced with a free energy of 0.65 eV, giving Nb-sTCNQ excellent catalytic cycle performance. The high catalytic activity of the two materials might be attributed to the effective charge transfer between the active center and adsorbed N2, which enables the active center to adsorb and activate inert N2 molecules well, and the reduction processes require small energy input (i.e., the maximum free energy changes are small). This work provides insights for finding highly efficient, stable, and low-cost nitrogen reduction reaction electrocatalysts. We hope our results can promote further experimental and theoretical research of this field.,我们首次采用密度泛函系统研究了二维单过渡金属锚定方形四氰基醌二甲烷单分子层(标记为:TM-sTCNQ,TM = 3d, 4d, 5d 系列过渡金属)的结构和电化学氮还原反应性质。理论方法。通过高通量筛选和全反应路径研究,获得了两种有前景的电化学氮还原反应催化剂Nb-sTCNQ和Mo-sTCNQ。Nb-sTCNQ上的氮还原反应起始电位低至-0.48 V。此外,Nb-sTCNQ催化剂可以快速解吸NH 3产生的自由能为 0.65 eV,使 Nb-sTCNQ 具有优异的催化循环性能。这两种材料的高催化活性可能归因于活性中心和吸附的N 2之间的有效电荷转移,这使得活性中心能够很好地吸附和活化惰性N 2分子,并且还原过程需要较小的能量输入(即,最大自由能变化很小)。这项工作为寻找高效、稳定和低成本的氮还原反应电催化剂提供了新思路。我们希望我们的研究结果能够促进该领域进一步的实验和理论研究。

"点击查看英文标题和摘要"
更新日期:2021-09-16

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号