当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Molecular Understanding of the Solid Interface-Induced Microstructures of 1-Hexyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide in Gas Absorption
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-03-07 , DOI: 10.1021/acs.iecr.1c05043 Guangzheng Jin 1 , Xinyao Song 1 , Qingwei Gao 2 , Yumeng Zhang 1 , Yifeng Chen 1 , Xiaohua Lu 1 , Yudan Zhu 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-03-07 , DOI: 10.1021/acs.iecr.1c05043 Guangzheng Jin 1 , Xinyao Song 1 , Qingwei Gao 2 , Yumeng Zhang 1 , Yifeng Chen 1 , Xiaohua Lu 1 , Yudan Zhu 1
Affiliation
|
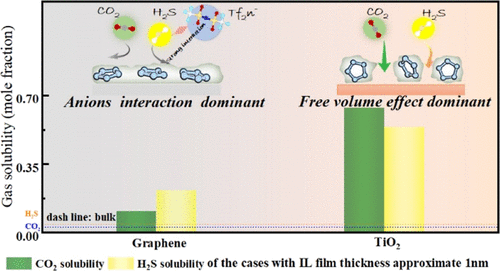
The abnormal intensification of gas absorption in nanoconfined ionic liquid (IL) systems has been receiving ever-increasing attention. In this work, grand canonical Monte Carlo and molecular dynamics simulations were performed for the systematic investigation of CO2 and H2S absorption by 1-hexyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide nanoconfined within different slits [graphene and rutile(110)]. The absorption mechanisms within different slits were greatly dependent on the solid interface-induced microstructures (spatial distribution and molecular orientation) of ILs. Within graphene slits, imidazole rings were mainly oriented parallel to the solid substrate, and IL stacking tightened such that gas absorption was dominated by the effect of the anions of ILs. By contrast, within rutile slits, the imidazole rings of ILs were mainly tilted on the solid surface because of the interfacial interaction. This orientation accounted for the large free volume that dominated the intensification of the absorption of both gases.
中文翻译:

1-Hexyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide 在气体吸收中固体界面诱导微观结构的分子理解
纳米限制离子液体 (IL) 系统中气体吸收的异常增强已受到越来越多的关注。在这项工作中,为系统研究 CO 2和 H 2进行了大规范 Monte Carlo 和分子动力学模拟1-己基-3-甲基咪唑鎓双(三氟甲基磺酰基)亚胺纳米限制在不同狭缝内的 S 吸收 [石墨烯和金红石 (110)]。不同狭缝内的吸收机制很大程度上取决于ILs的固体界面诱导的微观结构(空间分布和分子取向)。在石墨烯狭缝内,咪唑环主要平行于固体基板取向,并且离子液体堆叠收紧,使得气体吸收主要受离子液体阴离子的影响。相比之下,在金红石狭缝内,由于界面相互作用,ILs 的咪唑环主要在固体表面上倾斜。这种取向解释了主导两种气体吸收增强的大自由体积。
更新日期:2022-03-07
中文翻译:

1-Hexyl-3-methylimidazolium Bis(trifluoromethylsulfonyl)imide 在气体吸收中固体界面诱导微观结构的分子理解
纳米限制离子液体 (IL) 系统中气体吸收的异常增强已受到越来越多的关注。在这项工作中,为系统研究 CO 2和 H 2进行了大规范 Monte Carlo 和分子动力学模拟1-己基-3-甲基咪唑鎓双(三氟甲基磺酰基)亚胺纳米限制在不同狭缝内的 S 吸收 [石墨烯和金红石 (110)]。不同狭缝内的吸收机制很大程度上取决于ILs的固体界面诱导的微观结构(空间分布和分子取向)。在石墨烯狭缝内,咪唑环主要平行于固体基板取向,并且离子液体堆叠收紧,使得气体吸收主要受离子液体阴离子的影响。相比之下,在金红石狭缝内,由于界面相互作用,ILs 的咪唑环主要在固体表面上倾斜。这种取向解释了主导两种气体吸收增强的大自由体积。































 京公网安备 11010802027423号
京公网安备 11010802027423号