当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triiodide-in-Iodine Networks Stabilized by Quaternary Ammonium Cations as Accelerants for Electrode Kinetics of Iodide Oxidation in Aqueous Media
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-03-07 , DOI: 10.1021/acsami.1c21429 Hyeonmin Kim 1 , Kyung Mi Kim 2 , Jungju Ryu 1 , Sehyeok Ki 1 , Daewon Sohn 1 , Junghyun Chae 2 , Jinho Chang 1, 3
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2022-03-07 , DOI: 10.1021/acsami.1c21429 Hyeonmin Kim 1 , Kyung Mi Kim 2 , Jungju Ryu 1 , Sehyeok Ki 1 , Daewon Sohn 1 , Junghyun Chae 2 , Jinho Chang 1, 3
Affiliation
|
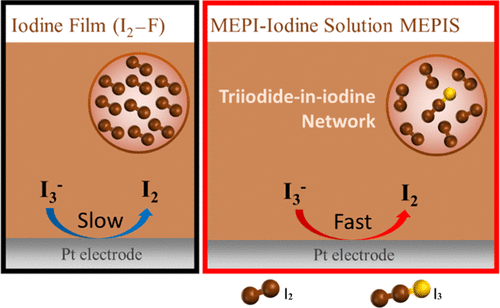
The Zn–polyiodide redox flow battery is considered to be a promising aqueous energy storage system. However, in its charging process, the electrode kinetics of I– oxidation often suffer from an intrinsically generated iodine film (I2–F) on the cathode of the battery. Therefore, it is critical to both understand and enhance the observed slow electrode kinetics of I– oxidation by an electrochemically generated I2–F. In this article, we introduced an electrogenerated N-methyl-N-ethyl pyrrolidinium iodide (MEPI)–iodine (I2) solution, designated as MEPIS, and demonstrated that the electrode kinetics of I– oxidation were dramatically enhanced compared to an I2–F under conventional electrolyte conditions, such as NaI. We showed that this result mainly contributed to the fast electro-oxidation of triiodide (I3–), which exists in the shape of a I3–-in-I2 network, [I3–·(I2)n]. Raman spectroscopic and electrochemical analyses showed that the composition of electrogenerated MEPIS changed from I3– to [I3–·(I2)n] via I5– as the anodic overpotential increased. We also confirmed that I– was electrochemically oxidized on a MEPIS-modified Pt electrode with fast electrode kinetics, which is clearly contrary to the nature of an I2–F derived from a NaI solution as a kinetic barrier of I– oxidation. Through stochastic MEPIS–particle impact electrochemistry and electrochemical impedance spectroscopy, we revealed that the enhanced electrode kinetics of I– oxidation in MEPIS can be attributed to the facilitated charge transfer of I3– oxidation in [I3–·(I2)n]. In addition, we found that the degree of freedom of I3– in a quaternary ammonium-based I2–F can also be critical to determine the kinetics of the electro-oxidation of I–, which is that MEPIS showed more enhanced charge-transfer kinetics of I– oxidation compared to tetrabutylammonium I3– due to the higher degree of freedom of I3–.
中文翻译:

季铵阳离子作为水介质中碘化物氧化电极动力学促进剂稳定的碘中三碘化物网络
Zn-聚碘化物氧化还原液流电池被认为是一种很有前途的水性储能系统。然而,在其充电过程中,I-氧化的电极动力学通常受到电池阴极上固有生成的碘膜(I 2 -F)的影响。因此,理解和增强观察到的由电化学产生的 I 2 -F氧化的 I 的慢电极动力学至关重要。在本文中,我们介绍了一种电生成的N-甲基-N-乙基吡咯烷碘化物 (MEPI)-碘 (I 2 ) 溶液,称为 MEPIS,并证明了 I -与传统电解质条件(例如 NaI)下的 I 2 -F相比,氧化显着增强。我们表明,该结果主要促成了三碘化物(I 3 - )的快速电氧化,三碘化物(I 3 -)以I 3 -in-I 2 网络的形式存在, [ I 3 - · ( I 2 ) n ]。拉曼光谱和电化学分析表明,电生成的MEPIS的组成通过I 5 -由I 3 -变为[I 3 - ·(I 2 ) n ]。随着阳极过电位的增加。我们还证实,I -在 MEPIS 修饰的 Pt 电极上被电化学氧化,具有快速的电极动力学,这显然与源自 NaI 溶液的 I 2 -F 作为 I -氧化的动力学屏障的性质相反。通过随机MEPIS-粒子冲击电化学和电化学阻抗谱,我们揭示了MEPIS中I-氧化的增强电极动力学可归因于[I 3 - · (I 2 ) n ]中I 3-氧化的促进电荷转移。 . 此外,我们发现 I 3的自由度–在基于季铵的 I 2 –F 中也可能对确定 I –的电氧化动力学至关重要,即与四丁基铵 I 3相比,MEPIS 显示出更强的 I –氧化电荷转移动力学–由于到 I 3 –的更高自由度。
更新日期:2022-03-07
中文翻译:

季铵阳离子作为水介质中碘化物氧化电极动力学促进剂稳定的碘中三碘化物网络
Zn-聚碘化物氧化还原液流电池被认为是一种很有前途的水性储能系统。然而,在其充电过程中,I-氧化的电极动力学通常受到电池阴极上固有生成的碘膜(I 2 -F)的影响。因此,理解和增强观察到的由电化学产生的 I 2 -F氧化的 I 的慢电极动力学至关重要。在本文中,我们介绍了一种电生成的N-甲基-N-乙基吡咯烷碘化物 (MEPI)-碘 (I 2 ) 溶液,称为 MEPIS,并证明了 I -与传统电解质条件(例如 NaI)下的 I 2 -F相比,氧化显着增强。我们表明,该结果主要促成了三碘化物(I 3 - )的快速电氧化,三碘化物(I 3 -)以I 3 -in-I 2 网络的形式存在, [ I 3 - · ( I 2 ) n ]。拉曼光谱和电化学分析表明,电生成的MEPIS的组成通过I 5 -由I 3 -变为[I 3 - ·(I 2 ) n ]。随着阳极过电位的增加。我们还证实,I -在 MEPIS 修饰的 Pt 电极上被电化学氧化,具有快速的电极动力学,这显然与源自 NaI 溶液的 I 2 -F 作为 I -氧化的动力学屏障的性质相反。通过随机MEPIS-粒子冲击电化学和电化学阻抗谱,我们揭示了MEPIS中I-氧化的增强电极动力学可归因于[I 3 - · (I 2 ) n ]中I 3-氧化的促进电荷转移。 . 此外,我们发现 I 3的自由度–在基于季铵的 I 2 –F 中也可能对确定 I –的电氧化动力学至关重要,即与四丁基铵 I 3相比,MEPIS 显示出更强的 I –氧化电荷转移动力学–由于到 I 3 –的更高自由度。































 京公网安备 11010802027423号
京公网安备 11010802027423号