当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Regio- and Stereoselective Formation of Azocan-2-ones via 8-EndoCyclization of α-Carbamoyl Radicals
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-02-24 , DOI: 10.1021/ja9082649 Xinqiang Fang 1 , Kun Liu 1 , Chaozhong Li 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-02-24 , DOI: 10.1021/ja9082649 Xinqiang Fang 1 , Kun Liu 1 , Chaozhong Li 1
Affiliation
|
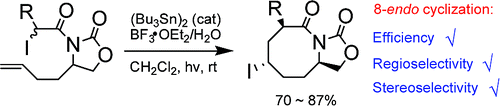
The iodine-atom-transfer 8-endo cyclization of alpha-carbamoyl radicals was investigated experimentally and theoretically. With the aid of Mg(ClO(4))(2) and a bis(oxazoline) ligand, N-ethoxycarbonyl-substituted N-(pent-4-enyl)-2-iodoalkanamides underwent 8-endo cyclization leading to the formation of only the corresponding 3,5-trans-substituted azocan-2-ones in excellent yields. Similarly, the BF(3).OEt(2)/H(2)O-promoted reactions of N-ethoxycarbonyl-N-(2-allylaryl)-2-iodoalkanamides afforded exclusively the benzazocanone products with a 3,5-cis configuration in high yields. The bidentate chelation of substrate radicals not only significantly improved the efficiency of cyclization but also resulted in the change of stereochemistry of azocanone products from 3,8-trans to 3,8-cis. Theoretical calculations at the UB3LYP/6-31G* level revealed that the cyclization of N-carbonyl-substituted alpha-carbamoyl radicals occurs via the E-conformational transition states without the presence of a Lewis acid. On the other hand, the cyclization proceeds via the Z-conformational transition states when the substrates form the bidentate chelation with a Lewis acid. In both cases, the 8-endo cyclization is always fundamentally preferred over the corresponding 7-exo cyclization. The complexed radicals having the more rigid conformations also allow the better stereochemical control in the iodine-atom-abstraction step. To further understand the reactivity of alpha-carbamoyl radicals, the competition between the 8-endo and 5-exo cyclization was also studied. The results demonstrated that the 8-endo cyclization is of comparable rate to the corresponding 5-exo cyclization for alpha-carbamoyl radicals with fixed Z-conformational transition states. As a comparison, the 8-endo mode is fundamentally preferred over the 5-exo mode in the cyclization of NH-amide substrates because the latter requires the Z-conformational transition states, whereas the former proceeds via the more stable E-conformational transition states.
中文翻译:

通过 α-氨基甲酰基自由基的 8-内环化有效区域选择性和立体选择性形成 Azocan-2-ones
通过实验和理论研究了 α-氨基甲酰基自由基的碘原子转移 8 内环化。在 Mg(ClO(4))(2) 和双(恶唑啉)配体的帮助下,N-乙氧羰基取代的 N-(pent-4-enyl)-2-iodoalkanamides 进行了 8-endo 环化,导致形成只有相应的 3,5-反式取代的 azocan-2-ones 产率高。类似地,BF(3).OEt(2)/H(2)O 促进的 N-乙氧羰基-N-(2-烯丙基芳基)-2-碘代烷酰胺反应仅提供具有 3,5-顺式构型的苯并环酮产物高产。底物自由基的双齿螯合不仅显着提高了环化效率,而且导致偶氮酮产物的立体化学从 3,8-反式变为 3,8-顺式。UB3LYP/6-31G* 水平的理论计算表明,N-羰基取代的 α-氨基甲酰基自由基的环化通过 E-构象过渡态发生,而无需路易斯酸。另一方面,当底物与路易斯酸形成双齿螯合时,环化通过 Z 构象过渡态进行。在这两种情况下,8-内环化总是优于相应的 7-外环化。具有更刚性构象的络合基团还允许在碘原子提取步骤中更好地控制立体化学。为了进一步了解 α-氨基甲酰基自由基的反应性,还研究了 8-endo 和 5-exo 环化之间的竞争。结果表明,对于具有固定 Z-构象过渡态的 α-氨基甲酰基自由基,8-内环化的速率与相应的 5-外环化速率相当。作为比较,在 NH-酰胺底物的环化中,8-endo 模式从根本上优于 5-exo 模式,因为后者需要 Z-构象过渡态,而前者通过更稳定的 E-构象过渡态进行.
更新日期:2010-02-24
中文翻译:

通过 α-氨基甲酰基自由基的 8-内环化有效区域选择性和立体选择性形成 Azocan-2-ones
通过实验和理论研究了 α-氨基甲酰基自由基的碘原子转移 8 内环化。在 Mg(ClO(4))(2) 和双(恶唑啉)配体的帮助下,N-乙氧羰基取代的 N-(pent-4-enyl)-2-iodoalkanamides 进行了 8-endo 环化,导致形成只有相应的 3,5-反式取代的 azocan-2-ones 产率高。类似地,BF(3).OEt(2)/H(2)O 促进的 N-乙氧羰基-N-(2-烯丙基芳基)-2-碘代烷酰胺反应仅提供具有 3,5-顺式构型的苯并环酮产物高产。底物自由基的双齿螯合不仅显着提高了环化效率,而且导致偶氮酮产物的立体化学从 3,8-反式变为 3,8-顺式。UB3LYP/6-31G* 水平的理论计算表明,N-羰基取代的 α-氨基甲酰基自由基的环化通过 E-构象过渡态发生,而无需路易斯酸。另一方面,当底物与路易斯酸形成双齿螯合时,环化通过 Z 构象过渡态进行。在这两种情况下,8-内环化总是优于相应的 7-外环化。具有更刚性构象的络合基团还允许在碘原子提取步骤中更好地控制立体化学。为了进一步了解 α-氨基甲酰基自由基的反应性,还研究了 8-endo 和 5-exo 环化之间的竞争。结果表明,对于具有固定 Z-构象过渡态的 α-氨基甲酰基自由基,8-内环化的速率与相应的 5-外环化速率相当。作为比较,在 NH-酰胺底物的环化中,8-endo 模式从根本上优于 5-exo 模式,因为后者需要 Z-构象过渡态,而前者通过更稳定的 E-构象过渡态进行.

































 京公网安备 11010802027423号
京公网安备 11010802027423号