当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective Oxidation of Furfural to Succinic Acid over Lewis Acidic Sn-Beta
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-03-04 , DOI: 10.1021/acscatal.1c05348 Yayati Naresh Palai 1, 2 , Abhijit Shrotri 1 , Atsushi Fukuoka 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2022-03-04 , DOI: 10.1021/acscatal.1c05348 Yayati Naresh Palai 1, 2 , Abhijit Shrotri 1 , Atsushi Fukuoka 1
Affiliation
|
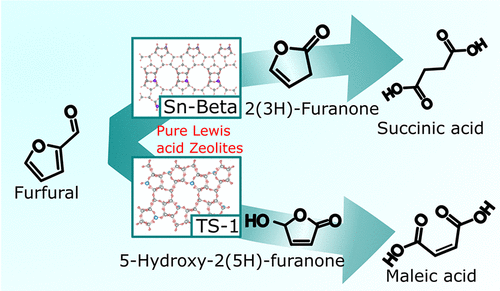
Selective production of succinic acid from furfural with H2O2 over Sn-Beta, a pure Lewis acid catalyst, is reported. Under optimized reaction conditions, 53% yield of succinic acid was obtained, and the catalyst was recyclable. 2(3H)-Furanone was detected as an intermediate using 1H nuclear magnetic resonance (NMR), HH correlation NMR spectroscopy, liquid chromatography–mass spectrometry (MS) and gas chromatography–MS. Kinetic modeling revealed that Baeyer–Villiger oxidation of furfural to 2(3H)-furanone was accelerated in comparison to other competing reactions in the presence of the pure Lewis acidic Sn-Beta catalyst. The Lewis acid density of the Sn-Beta catalyst was directly correlated to the formation rate of products, confirming a Lewis acid-catalyzed mechanism. Detailed characterization showed that Sn-Beta activates furfural by coordinating to the carbonyl group and does not activate H2O2. On the other hand, the parent HBeta-38 zeolite produced activated H2O2 in solution, which caused side reactions to produce maleic acid. Selectivity of Sn-Beta was also compared with that of TS-1, another Lewis acid zeolite, which produced maleic acid because of the ability of TS-1 to activate H2O2 as a hydroperoxy species. Therefore, Sn-Beta is a selective and reusable catalyst for succinic acid synthesis from biomass-derived furfural.
中文翻译:

路易斯酸性 Sn-β 选择性氧化糠醛制琥珀酸
报道了在纯路易斯酸催化剂 Sn-Beta 上由糠醛与 H 2 O 2选择性生产琥珀酸。在优化的反应条件下,丁二酸的收率为53%,催化剂可回收利用。使用1 H 核磁共振 (NMR)、HH 相关 NMR 光谱、液相色谱-质谱 (MS) 和气相色谱-MS 检测2(3 H )-呋喃酮作为中间体。动力学模型显示,糠醛的 Baeyer-Villiger 氧化为 2(3 H)-呋喃酮与纯路易斯酸性 Sn-β 催化剂存在下的其他竞争反应相比得到加速。Sn-Beta 催化剂的路易斯酸密度与产物的形成速率直接相关,证实了路易斯酸催化的机理。详细表征表明,Sn-Beta 通过与羰基配位来激活糠醛,但不会激活 H 2 O 2。另一方面,母体HBeta-38沸石在溶液中产生活化的H 2 O 2,这引起副反应产生马来酸。Sn-Beta 的选择性也与 TS-1 的选择性进行了比较,TS-1 是另一种路易斯酸沸石,由于 TS-1 具有活化 H 2 O的能力而产生马来酸2作为氢过氧化物。因此,Sn-Beta 是一种用于从生物质衍生的糠醛合成琥珀酸的选择性和可重复使用的催化剂。
更新日期:2022-03-04
中文翻译:

路易斯酸性 Sn-β 选择性氧化糠醛制琥珀酸
报道了在纯路易斯酸催化剂 Sn-Beta 上由糠醛与 H 2 O 2选择性生产琥珀酸。在优化的反应条件下,丁二酸的收率为53%,催化剂可回收利用。使用1 H 核磁共振 (NMR)、HH 相关 NMR 光谱、液相色谱-质谱 (MS) 和气相色谱-MS 检测2(3 H )-呋喃酮作为中间体。动力学模型显示,糠醛的 Baeyer-Villiger 氧化为 2(3 H)-呋喃酮与纯路易斯酸性 Sn-β 催化剂存在下的其他竞争反应相比得到加速。Sn-Beta 催化剂的路易斯酸密度与产物的形成速率直接相关,证实了路易斯酸催化的机理。详细表征表明,Sn-Beta 通过与羰基配位来激活糠醛,但不会激活 H 2 O 2。另一方面,母体HBeta-38沸石在溶液中产生活化的H 2 O 2,这引起副反应产生马来酸。Sn-Beta 的选择性也与 TS-1 的选择性进行了比较,TS-1 是另一种路易斯酸沸石,由于 TS-1 具有活化 H 2 O的能力而产生马来酸2作为氢过氧化物。因此,Sn-Beta 是一种用于从生物质衍生的糠醛合成琥珀酸的选择性和可重复使用的催化剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号