当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formation of Breslow Intermediates from N-Heterocyclic Carbenes and Aldehydes Involves Autocatalysis by the Breslow Intermediate, and a Hemiacetal
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-03-03 , DOI: 10.1002/anie.202117682 Alina Wessels 1 , Martin Klussmann 2, 3 , Martin Breugst 1 , Nils E Schlörer 1 , Albrecht Berkessel 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2022-03-03 , DOI: 10.1002/anie.202117682 Alina Wessels 1 , Martin Klussmann 2, 3 , Martin Breugst 1 , Nils E Schlörer 1 , Albrecht Berkessel 1
Affiliation
|
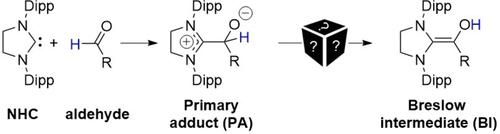
Under aprotic conditions, Breslow intermediates can be generated from N-heterocyclic carbenes such as imidazolidin-2-ylidenes ((hyphenation: “imidazol-idin-2-ylidenes”)) and aldehydes. But how is the intermediary “primary adduct” (PA) converted to the diaminoenol (BI)? Our kinetic study shows that the highly unfavorable 1,2-C-to-O H-shift is autocatalyzed ((hyphenation: “au-to-cata-lyzed”)) by the Breslow intermediate, and that a hemiacetal plays a crucial role in the presence of excess aldehyde.
中文翻译:

由 N-杂环卡宾和醛形成 Breslow 中间体涉及 Breslow 中间体和半缩醛的自催化
在非质子条件下,Breslow 中间体可以由 N-杂环卡宾生成,例如 imidazolidin-2-ylidenes((连字符:“imidazol-idin-2-ylidenes”))和醛。但是中间的“初级加合物”(PA)是如何转化为二氨基烯醇(BI)的呢?我们的动力学研究表明,高度不利的 1,2-C-to-O H-shift 被 Breslow 中间体自动催化((连字符:“自动催化”)),并且半缩醛起着至关重要的作用在过量醛的存在下。
更新日期:2022-03-03
中文翻译:

由 N-杂环卡宾和醛形成 Breslow 中间体涉及 Breslow 中间体和半缩醛的自催化
在非质子条件下,Breslow 中间体可以由 N-杂环卡宾生成,例如 imidazolidin-2-ylidenes((连字符:“imidazol-idin-2-ylidenes”))和醛。但是中间的“初级加合物”(PA)是如何转化为二氨基烯醇(BI)的呢?我们的动力学研究表明,高度不利的 1,2-C-to-O H-shift 被 Breslow 中间体自动催化((连字符:“自动催化”)),并且半缩醛起着至关重要的作用在过量醛的存在下。































 京公网安备 11010802027423号
京公网安备 11010802027423号