当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of [1,2,3]Triazolo-[1,5-a]quinoxalin-4(5H)-ones through Photoredox-Catalyzed [3 + 2] Cyclization Reactions with Hypervalent Iodine(III) Reagents
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-03-02 , DOI: 10.1021/acs.joc.2c00135 Jinxia Wen 1 , Wenyan Zhao 1 , Xu Gao 1 , Xiaofang Ren 1 , Chunping Dong 1 , Cheli Wang 1 , Li Liu 1 , Jian Li 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-03-02 , DOI: 10.1021/acs.joc.2c00135 Jinxia Wen 1 , Wenyan Zhao 1 , Xu Gao 1 , Xiaofang Ren 1 , Chunping Dong 1 , Cheli Wang 1 , Li Liu 1 , Jian Li 1
Affiliation
|
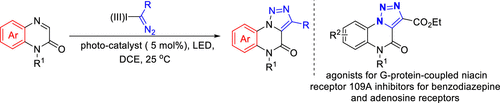
An efficient synthesis of a variety of [1,2,3]triazolo-[1,5-a]quinoxalin-4(5H)-ones via a [3 + 2] cyclization reaction by photoredox catalysis between quinoxalinones and hypervalent iodine(III) reagents is reported. A range of quinoxalinones and hypervalent iodine(III) reagents were tolerated well. This cyclization reaction allows access to structurally diverse [1,2,3]triazolo-[1,5-a]quinoxalin-4(5H)-ones in moderate to good yields.
中文翻译:

[1,2,3]Triazolo-[1,5-a]quinoxalin-4(5H)-ones 通过光氧化还原催化的 [3 + 2] 环化反应与高价碘 (III) 试剂的合成
通过喹喔啉酮和高价碘之间的光氧化还原催化 [3 + 2] 环化反应高效合成多种 [1,2,3] 三唑-[1,5- a ]quinoxalin-4(5 H )-ones( III) 报告试剂。一系列喹喔啉酮和高价碘 (III) 试剂耐受性良好。这种环化反应允许以中等至良好的产率获得结构多样的 [1,2,3]三唑-[1,5- a ]喹喔啉-4(5 H )-酮。
更新日期:2022-03-02
中文翻译:

[1,2,3]Triazolo-[1,5-a]quinoxalin-4(5H)-ones 通过光氧化还原催化的 [3 + 2] 环化反应与高价碘 (III) 试剂的合成
通过喹喔啉酮和高价碘之间的光氧化还原催化 [3 + 2] 环化反应高效合成多种 [1,2,3] 三唑-[1,5- a ]quinoxalin-4(5 H )-ones( III) 报告试剂。一系列喹喔啉酮和高价碘 (III) 试剂耐受性良好。这种环化反应允许以中等至良好的产率获得结构多样的 [1,2,3]三唑-[1,5- a ]喹喔啉-4(5 H )-酮。






























 京公网安备 11010802027423号
京公网安备 11010802027423号