当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(PNP)Cobalt-Catalyzed Olefination of Diazoalkanes
Organometallics ( IF 2.5 ) Pub Date : 2022-03-02 , DOI: 10.1021/acs.organomet.1c00688 Sam Yruegas 1 , Scott P. Semproni 1 , Paul J. Chirik 1
Organometallics ( IF 2.5 ) Pub Date : 2022-03-02 , DOI: 10.1021/acs.organomet.1c00688 Sam Yruegas 1 , Scott P. Semproni 1 , Paul J. Chirik 1
Affiliation
|
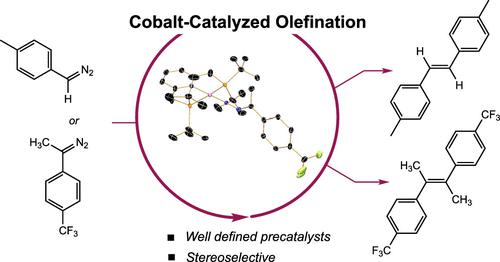
Addition of excess diazoalkane to the pincer-supported cobalt(I) dinitrogen complex (tBumPNP)CoN2 (tBumPNP = modified 2,6-bis[(ditert-butylphosphino)methyl]pyridine) resulted in the catalytic formation of the homocoupled alkene product with concomitant loss of N2. Monosubstituted diazoalkanes, trimethylsilyldiazomethane and tolyldiazomethane, generated the olefin product in quantitative yield with exclusive (E)-stereoselectivity. Disubstituted diazoalkanes, diphenyldiazomethane and 9-diazofluorene, also yielded the olefin as the major product along with minor azine coupling. Investigations into the nature of the diazoalkane–cobalt interaction by multinuclear NMR spectroscopy and X-ray diffraction established end-on diazoalkane cobalt complexes as the resting states. The isolated four-coordinate cobalt diazoalkane complexes promoted conversion to the corresponding olefin. The reaction of (tBumPNP)CoN2 with an α-diazo-β-ketoester resulted in the formation of a five-coordinate Co(I)-diazoalkane complex with a chelating ester unit that was unreactive for olefination.
中文翻译:

(PNP)钴催化重氮烷烃的烯烃化
向钳形负载的钴 (I) 二氮络合物 ( tBu mPNP)CoN 2 ( tBu mPNP = modified 2,6-bis[(di tert -butylphosphino )methyl]pyridine) 添加过量的重氮烷导致催化形成同源偶联伴随 N 2损失的烯烃产物。单取代的重氮烷烃、三甲基甲硅烷基重氮甲烷和甲苯基重氮甲烷以定量收率生成烯烃产物,具有独特的 ( E)-立体选择性。二取代的重氮烷烃、二苯基重氮甲烷和 9-重氮芴也产生作为主要产物的烯烃以及少量吖嗪偶联。通过多核 NMR 光谱和 X 射线衍射对重氮烷-钴相互作用的性质进行研究,建立了重氮烷钴络合物作为静止状态。孤立的四配位钴重氮烷络合物促进转化为相应的烯烃。( tBu mPNP)CoN 2与 α-重氮-β-酮酯的反应导致形成五配位 Co(I)-重氮烷络合物,其螯合酯单元对烯化反应不活跃。
更新日期:2022-03-02
中文翻译:

(PNP)钴催化重氮烷烃的烯烃化
向钳形负载的钴 (I) 二氮络合物 ( tBu mPNP)CoN 2 ( tBu mPNP = modified 2,6-bis[(di tert -butylphosphino )methyl]pyridine) 添加过量的重氮烷导致催化形成同源偶联伴随 N 2损失的烯烃产物。单取代的重氮烷烃、三甲基甲硅烷基重氮甲烷和甲苯基重氮甲烷以定量收率生成烯烃产物,具有独特的 ( E)-立体选择性。二取代的重氮烷烃、二苯基重氮甲烷和 9-重氮芴也产生作为主要产物的烯烃以及少量吖嗪偶联。通过多核 NMR 光谱和 X 射线衍射对重氮烷-钴相互作用的性质进行研究,建立了重氮烷钴络合物作为静止状态。孤立的四配位钴重氮烷络合物促进转化为相应的烯烃。( tBu mPNP)CoN 2与 α-重氮-β-酮酯的反应导致形成五配位 Co(I)-重氮烷络合物,其螯合酯单元对烯化反应不活跃。











































 京公网安备 11010802027423号
京公网安备 11010802027423号