Cell Reports ( IF 7.5 ) Pub Date : 2022-03-01 , DOI: 10.1016/j.celrep.2022.110453 Neali Armstrong 1 , Claire M Storey 1 , Sarah E Noll 2 , Katherine Margulis 2 , Myat Han Soe 1 , Haixia Xu 1 , Benjamin Yeh 3 , Lauren Fishbein 4 , Electron Kebebew 5 , Brooke E Howitt 6 , Richard N Zare 2 , Julien Sage 7 , Justin P Annes 8
|
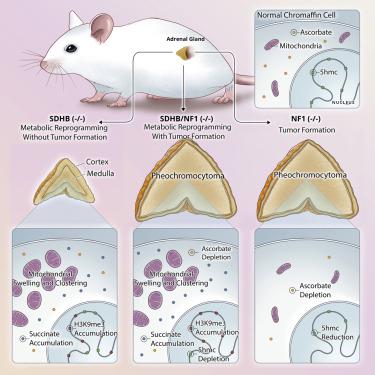
Inherited pathogenic succinate dehydrogenase (SDHx) gene mutations cause the hereditary pheochromocytoma and paraganglioma tumor syndrome. Syndromic tumors exhibit elevated succinate, an oncometabolite that is proposed to drive tumorigenesis via DNA and histone hypermethylation, mitochondrial expansion, and pseudohypoxia-related gene expression. To interrogate this prevailing model, we disrupt mouse adrenal medulla SDHB expression, which recapitulates several key molecular features of human SDHx tumors, including succinate accumulation but not 5hmC loss, HIF accumulation, or tumorigenesis. By contrast, concomitant SDHB and the neurofibromin 1 tumor suppressor disruption yields SDHx-like pheochromocytomas. Unexpectedly, in vivo depletion of the 2-oxoglutarate (2-OG) dioxygenase cofactor ascorbate reduces SDHB-deficient cell survival, indicating that SDHx loss may be better tolerated by tissues with high antioxidant capacity. Contrary to the prevailing oncometabolite model, succinate accumulation and 2-OG-dependent dioxygenase inhibition are insufficient for mouse pheochromocytoma tumorigenesis, which requires additional growth-regulatory pathway activation.
中文翻译:

SDHB 敲除和琥珀酸积累不足以导致肿瘤发生,但 SDHB/NF1 双重缺失会产生 SDHx 样嗜铬细胞瘤
遗传性致病性琥珀酸脱氢酶 ( SDHx ) 基因突变导致遗传性嗜铬细胞瘤和副神经节瘤肿瘤综合征。综合征性肿瘤表现出升高的琥珀酸盐,这是一种致癌代谢物,被认为通过 DNA 和组蛋白高甲基化、线粒体扩张和假缺氧相关基因表达来驱动肿瘤发生。为了审视这个流行的模型,我们破坏了小鼠肾上腺髓质 SDHB 的表达,它概括了人类 SDHx 肿瘤的几个关键分子特征,包括琥珀酸积累而不是 5hmC 丢失、HIF 积累或肿瘤发生。相比之下,伴随的 SDHB 和神经纤维瘤蛋白 1 肿瘤抑制因子破坏会产生 SDHx 样嗜铬细胞瘤。没想到,体内2-酮戊二酸 (2-OG) 双加氧酶辅助因子抗坏血酸的消耗会降低 SDHB 缺陷细胞的存活率,表明具有高抗氧化能力的组织可能更好地耐受 SDHx 损失。与流行的致癌代谢物模型相反,琥珀酸积累和 2-OG 依赖性双加氧酶抑制不足以促进小鼠嗜铬细胞瘤的发生,这需要额外的生长调节通路激活。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号