Synthesis ( IF 2.2 ) Pub Date : 2022-01-14 , DOI: 10.1055/a-1740-5785 Jun Dong 1 , Youwei Chen 1 , Xingcai Huang 1
|
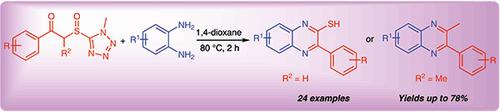
A series of quinoxaline-2-thiols and quinoxalines were prepared in moderate to good yields from various phenacyl sulfoxides bearing 1-methyl-1H-tetrazole and o-arylenediamines. The proposed reaction mechanism involves generation of sulfines from the phenacyl sulfoxides bearing 1-methyl-1H-tetrazole through thermolysis elimination. Then, site-selective carbophilic addition of sulfines by o-arylenediamines, followed by elimination, intramolecular nucleophilic addition, and dehydration condensation. The current method provides a direct and simple strategy for the preparation of quinoxaline-2-thiols and quinoxalines.
中文翻译:

由 α-氧代亚砜和邻亚芳基二胺轻松合成喹喔啉-2-硫醇和喹喔啉
从各种带有 1-甲基-1 H-四唑和邻亚芳基二胺的苯甲酰亚砜中制备出一系列的 quinoxaline-2-thiol 和 quinoxalines,产率适中到高。所提出的反应机理涉及通过热解消除从带有 1-甲基-1 H-四唑的苯甲酰基亚砜中生成亚磺酸。然后,邻亚芳基二胺对亚磺酸进行位点选择性亲碳加成,然后进行消除、分子内亲核加成和脱水缩合。目前的方法为制备喹喔啉-2-硫醇和喹喔啉提供了一种直接而简单的策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号