Chinese Chemical Letters ( IF 9.4 ) Pub Date : 2022-03-01 , DOI: 10.1016/j.cclet.2022.02.069
Rugeng Liu 1 , Yangyang Meng 1 , Wenjing Ji 1 , Wei Han 1 , Mei Li 1 , Yang Sun 1
|
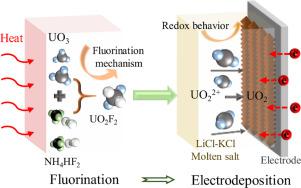
In this work, a technique was proposed to prepare UO2 from UO3 by the two processes of fluorination reaction of UO3 with NH4HF2 and electrochemical reduction of for the recycle uranium. The feasibility of fluorination reaction was firstly confirmed using thermodynamic calculation; then, the products were analyzed using XRD, Raman and fluorescence to be UO2F2. The fluorination mechanism was inferred to be UO3(s) + NH4HF2 → (NH4)3UO2F5→ NH4(UO2)2F5 → UO2F2. The redox behavior of on W electrode was investigated by cyclic voltammetry and square wave voltammetry, which indicated that was reduced to UO2 via a two-step single electron transfer with diffusion-controlled. The diffusion coefficient of was calculated to be 6.22 × 10−5 cm2/s. The disproportionation reaction of was observed, and the relationship between the disproportionation reaction and scan rate was discussed. Moreover, the electrochemical fabrication of UO2 was conducted by electrolysis at −0.8 V, and the product was analyzed by XRD, SEM and EDS to be UO2. ICP-AES results showed that the extraction efficiency of UO2 could reach 98.53%.
中文翻译:

UO3的氟化反应及UO2的电化学制备
在这项工作中,提出了一种通过UO 3与NH 4 HF 2的氟化反应和电化学还原UO 3两个过程来制备UO 2的技术。用于回收铀。首次通过热力学计算证实了氟化反应的可行性;然后,使用XRD、拉曼和荧光分析产物为UO 2 F 2。推测其氟化机理为UO 3 (s) + NH 4 HF 2 → (NH 4 ) 3 UO 2 F 5 → NH 4 (UO 2 ) 2 F 5 → UO 2 F 2。的氧化还原行为用循环伏安法和方波伏安法研究了 W 电极上的通过具有扩散控制的两步单电子转移将其还原为UO 2 。 扩散系数计算为6.22×10 -5 cm 2 /s。歧化反应观察,讨论歧化反应与扫描速率的关系。此外,在-0.8 V下通过电解进行UO 2的电化学制备,产物通过XRD、SEM和EDS分析为UO 2。ICP-AES结果表明,UO 2的提取效率可达98.53%。

































 京公网安备 11010802027423号
京公网安备 11010802027423号