当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Probing of Size-Selective Crown Ether Macrocycle Ligands for Transplutonium Element Separation
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-03-01 , DOI: 10.1021/acs.inorgchem.1c03853 Yang Liu 1, 2 , Cong-Zhi Wang 1 , Qun-Yan Wu 1 , Jian-Hui Lan 1 , Zhi-Fang Chai 1, 3 , Wang-Suo Wu 2 , Wei-Qun Shi 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2022-03-01 , DOI: 10.1021/acs.inorgchem.1c03853 Yang Liu 1, 2 , Cong-Zhi Wang 1 , Qun-Yan Wu 1 , Jian-Hui Lan 1 , Zhi-Fang Chai 1, 3 , Wang-Suo Wu 2 , Wei-Qun Shi 1
Affiliation
|
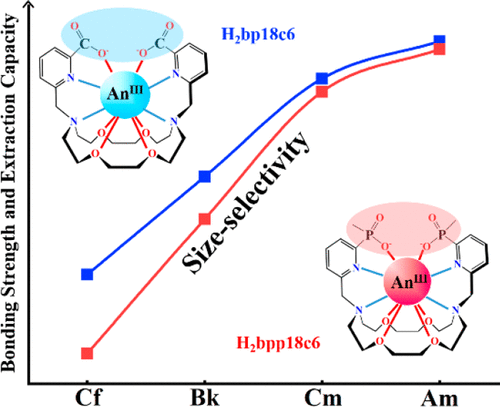
Effective separation and recovery of chemically similar transplutonium elements from adjacent actinides is extremely challenging in spent fuel reprocessing. Deep comprehension of the complexation of transplutonium elements and ligands is significant for the design and development of ligands for the in-group separation of transplutonium elements. Because of experimental difficulties of transplutonium elements, theoretical calculation has become an effective means of exploring transplutonium complexes. In this work, we systematically investigated the coordination mechanism between transplutonium elements (An = Am, Cm, Bk, Cf) and two crown ether macrocyclic ligands [N,N′- bis[(6-carboxy-2-pyridyl)methyl]-1,10-diaza-18-crown-6 (H2bp18c6) and N,N′-bis[(6-methylphosphinic-2-pyridyl)methyl]-1,10-diaza-18-crown-6 (H2bpp18c6)] through quasi-relativistic density functional theory. The extraction complexes of [Anbp18c6]+ and [Anbpp18c6]+ possess similar geometrical structures with actinide atoms located in the cavity of the ligands. Bonding nature analysis indicates that the coordination ability of the coordinating atoms in pendent arms is stronger than that in the crown ether macrocycle because of the limitation of the macrocycle. Most of the coordination atoms of the H2bp18c6 ligand have a stronger ability to coordinate with metal ions than those of the H2bpp18c6 ligand. In addition, the bonding strength between the metal ions and ligands gradually weakens from Am to Cf, which is mainly attributed to the size selectivity of the ligands. Thermodynamic analysis shows that the H2bp18c6 ligand has a stronger extraction capacity than the H2bpp18c6 ligand, while the H2bpp18c6 ligand is superior in terms of the in-group separation ability. The extraction capacity of the two ligands for metal ions gradually decreases across the actinide series, indicating that these crown ether macrocycle ligands have size selectivity for these actinide cations as a result of steric constraint of the crown ether ring. We hope that these results offer theoretical clues for the development of macrocycle ligands for in-group transplutonium separation.
中文翻译:

用于分离钚元素的尺寸选择性冠醚大环配体的理论探索
从相邻锕系元素中有效分离和回收化学相似的钚元素在乏燃料后处理中极具挑战性。深入理解跨钚元素和配体的络合对于设计和开发用于跨钚元素组内分离的配体具有重要意义。由于跨钚元素的实验困难,理论计算已成为探索跨钚配合物的有效手段。在这项工作中,我们系统地研究了钚元素(An = Am,Cm,Bk,Cf)与两个冠醚大环配体[ N,N'-双[(6-羧基-2-吡啶基)甲基]-之间的配位机制。 1,10-diaza-18-crown-6 (H 2 bp18c6) 和N, N'-bis[(6-methylphosphinic-2-pyridyl)methyl]-1,10- diaza -18-crown-6 (H 2 bpp18c6)] 通过准相对论密度泛函理论。[Anbp18c6] +和[Anbpp18c6] +的提取配合物具有相似的几何结构,锕系原子位于配体的空腔中。成键性分析表明,由于大环的限制,悬臂配位原子的配位能力强于冠醚大环。H 2 bp18c6 配体的大部分配位原子与金属离子的配位能力比H 2强bpp18c6 配体。此外,金属离子与配体的结合强度从Am到Cf逐渐减弱,这主要归因于配体的尺寸选择性。热力学分析表明,H 2 bp18c6 配体比 H 2 bpp18c6 配体具有更强的提取能力,而 H 2bpp18c6 配体在组内分离能力方面更胜一筹。两种配体对金属离子的萃取能力在整个锕系系中逐渐降低,表明这些冠醚大环配体对这些锕系阳离子具有尺寸选择性,这是由于冠醚环的空间约束。我们希望这些结果为开发用于组内钚分离的大环配体提供理论线索。
更新日期:2022-03-01
中文翻译:

用于分离钚元素的尺寸选择性冠醚大环配体的理论探索
从相邻锕系元素中有效分离和回收化学相似的钚元素在乏燃料后处理中极具挑战性。深入理解跨钚元素和配体的络合对于设计和开发用于跨钚元素组内分离的配体具有重要意义。由于跨钚元素的实验困难,理论计算已成为探索跨钚配合物的有效手段。在这项工作中,我们系统地研究了钚元素(An = Am,Cm,Bk,Cf)与两个冠醚大环配体[ N,N'-双[(6-羧基-2-吡啶基)甲基]-之间的配位机制。 1,10-diaza-18-crown-6 (H 2 bp18c6) 和N, N'-bis[(6-methylphosphinic-2-pyridyl)methyl]-1,10- diaza -18-crown-6 (H 2 bpp18c6)] 通过准相对论密度泛函理论。[Anbp18c6] +和[Anbpp18c6] +的提取配合物具有相似的几何结构,锕系原子位于配体的空腔中。成键性分析表明,由于大环的限制,悬臂配位原子的配位能力强于冠醚大环。H 2 bp18c6 配体的大部分配位原子与金属离子的配位能力比H 2强bpp18c6 配体。此外,金属离子与配体的结合强度从Am到Cf逐渐减弱,这主要归因于配体的尺寸选择性。热力学分析表明,H 2 bp18c6 配体比 H 2 bpp18c6 配体具有更强的提取能力,而 H 2bpp18c6 配体在组内分离能力方面更胜一筹。两种配体对金属离子的萃取能力在整个锕系系中逐渐降低,表明这些冠醚大环配体对这些锕系阳离子具有尺寸选择性,这是由于冠醚环的空间约束。我们希望这些结果为开发用于组内钚分离的大环配体提供理论线索。
















































 京公网安备 11010802027423号
京公网安备 11010802027423号