当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrochemical and Structural Behavior of Trirutile-Derived FeF3 During Sodiation and Desodiation
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-02-27 , DOI: 10.1021/acsaem.1c03756
Yayun Zheng 1 , Jinkwang Hwang 1, 2 , Kazuhiko Matsumoto 1, 2 , Rika Hagiwara 1, 2
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2022-02-27 , DOI: 10.1021/acsaem.1c03756
Yayun Zheng 1 , Jinkwang Hwang 1, 2 , Kazuhiko Matsumoto 1, 2 , Rika Hagiwara 1, 2
Affiliation
|
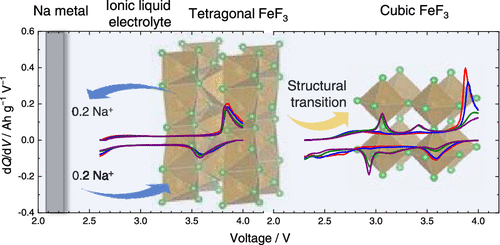
Despite significant advances in the Li–Fe–F systems for lithium-ion batteries, the investigation on the Na–Fe–F systems for sodium-ion batteries is still insufficient. Herein, trirutile-derived FeF3 prepared through the electrochemical delithiation of trirutile Li0.5FeF3 has been examined as the positive electrode of sodium-ion batteries at 90 °C with a thermally stable ionic liquid electrolyte to shed light on the structural evolutions occurring during sodiation–desodiation for the first time. Synchrotron X-ray diffraction revealed that the reversible topotactic extraction/insertion of 0.2 Na+ proceeds during cycling between 2.6 and 4.0 V, which triggers a two-phase reaction between tetragonal NaxFeF3 and tetragonal FeF3. A lower cutoff voltage of 2.3–4.0 V induces a partial structural transition from tetragonal FeF3 into cubic FeF3 along with cycling, where the topotactic Na+ extraction/insertion not only occurs in the tetragonal structure but also involves the reversible phase transformation between orthorhombic NaFeF3 and cubic FeF3 after several cycles.
中文翻译:

三金红石衍生的 FeF3 在钠化和脱钠过程中的电化学和结构行为
尽管用于锂离子电池的 Li-Fe-F 体系取得了重大进展,但对用于钠离子电池的 Na-Fe-F 体系的研究仍然不足。在此,通过三金红石 Li 0.5 FeF 3的电化学脱锂制备的三金红石衍生的 FeF 3作为钠离子电池的正极在 90 °C 与热稳定的离子液体电解质进行了研究,以阐明在此过程中发生的结构演变。钠化-第一次去钠化。同步加速器 X 射线衍射表明,在 2.6 和 4.0 V 之间的循环过程中,0.2 Na +的可逆拓扑结构提取/插入进行,这引发了四方 Na x FeF之间的两相反应3和四方FeF 3。2.3-4.0 V 的较低截止电压随着循环引起从四方 FeF 3到立方 FeF 3的部分结构转变,其中拓扑 Na +提取/插入不仅发生在四方结构中,而且还涉及正交晶系之间的可逆相变几个循环后的NaFeF 3和立方FeF 3 。
更新日期:2022-02-27
中文翻译:

三金红石衍生的 FeF3 在钠化和脱钠过程中的电化学和结构行为
尽管用于锂离子电池的 Li-Fe-F 体系取得了重大进展,但对用于钠离子电池的 Na-Fe-F 体系的研究仍然不足。在此,通过三金红石 Li 0.5 FeF 3的电化学脱锂制备的三金红石衍生的 FeF 3作为钠离子电池的正极在 90 °C 与热稳定的离子液体电解质进行了研究,以阐明在此过程中发生的结构演变。钠化-第一次去钠化。同步加速器 X 射线衍射表明,在 2.6 和 4.0 V 之间的循环过程中,0.2 Na +的可逆拓扑结构提取/插入进行,这引发了四方 Na x FeF之间的两相反应3和四方FeF 3。2.3-4.0 V 的较低截止电压随着循环引起从四方 FeF 3到立方 FeF 3的部分结构转变,其中拓扑 Na +提取/插入不仅发生在四方结构中,而且还涉及正交晶系之间的可逆相变几个循环后的NaFeF 3和立方FeF 3 。































 京公网安备 11010802027423号
京公网安备 11010802027423号