Journal of Molecular Structure ( IF 4.0 ) Pub Date : 2022-02-25 , DOI: 10.1016/j.molstruc.2022.132688 Mohamed Rbaa 1 , Sara Haida 1 , Burak Tuzun 2 , Abdelhadi hichar 3 , Anouar El Hassane 4 , Abderahim Kribii 1 , Younes Lakhrissi 1 , Taibi Ben Hadda 5, 6 , Abdelkader Zarrouk 7 , Brahim Lakhrissi 1 , Elyor Berdimurodov 8
|
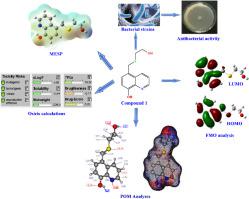
In this present research work, new 8-hydroxyquinoline derivatives were synthesized and their molecular structures were confirmed by the 1H-NMR, 13C-NMR, IR and elemental analysis. The antioxidant activity of the synthesized compounds is evaluated through their capacity to scavenge 2,2-di-phenyl-1-picrylhydrazyl (DPPH) free radicals. In addition, their antibacterial activity was examined against bacterial strains of S. aureus, K. pneumonia, and E. coli. The obtained results showed that the antibacterial activity of studied compounds for Gram-positive and Gram-negative is higher than Penicillin G. The theoretical properties of selected compounds was computed by the DFT method. POM analysis (Petra/Osiris/Molinspiration) of studied molecules has been done. Their biological activities were compared against breast, liver and lung cancer proteins. The obtained results showed the synthesized derivatives were effective antibacterial and antioxidant agents against various biological material.
中文翻译:

新型 8-羟基喹啉衍生物的合成、表征和生物活性:实验、分子对接、DFT 和 POM 分析
在本研究工作中,合成了新的 8-羟基喹啉衍生物,并通过1 H-NMR、13C-NMR、IR和元素分析。合成化合物的抗氧化活性通过其清除 2,2-二苯基-1-苦基肼 (DPPH) 自由基的能力来评估。此外,还检测了它们对金黄色葡萄球菌、肺炎克雷伯菌和大肠杆菌的抗菌活性。所得结果表明,所研究化合物对革兰氏阳性和革兰氏阴性菌的抗菌活性均高于青霉素G。所选化合物的理论性质采用DFT法计算。已完成研究分子的 POM 分析(Petra/Osiris/Molinspiration)。它们的生物活性与乳腺癌、肝癌和肺癌蛋白进行了比较。所得结果表明,合成的衍生物对各种生物材料具有有效的抗菌和抗氧化作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号