当前位置:
X-MOL 学术
›
Adv. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Triphasic Metal Oxide Photocatalyst for Reaction Site-Specific Production of Hydrogen Peroxide from Oxygen Reduction and Water Oxidation
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2022-02-25 , DOI: 10.1002/aenm.202104052 Keon‐Han Kim 1, 2 , Se‐Jun Kim 3 , Won Ho Choi 1 , Heebin Lee 1 , Byeong Cheul Moon 4 , Gi Hwan Kim 1 , Jae Won Choi 1 , Dong Gyu Park 1 , Jong Hui Choi 1 , Hyungjun Kim 3 , Jeung Ku Kang 1
Advanced Energy Materials ( IF 24.4 ) Pub Date : 2022-02-25 , DOI: 10.1002/aenm.202104052 Keon‐Han Kim 1, 2 , Se‐Jun Kim 3 , Won Ho Choi 1 , Heebin Lee 1 , Byeong Cheul Moon 4 , Gi Hwan Kim 1 , Jae Won Choi 1 , Dong Gyu Park 1 , Jong Hui Choi 1 , Hyungjun Kim 3 , Jeung Ku Kang 1
Affiliation
|
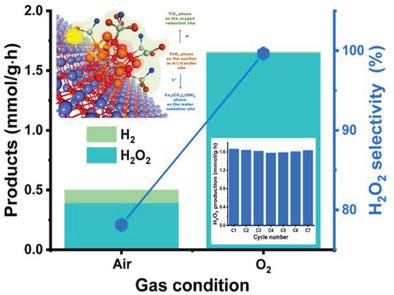
The search for photocatalysts allowing the highly active, selective, and stable conversion of molecular oxygen into hydrogen peroxide is of worldwide interest. Here, the authors report the efficient conversion of O2 into H2O2 with ≈100% selectivity and stable cycle stability by a triphasic metal oxide photocatalyst with a cobalt hydroxide carbonate nanosheet phase for water oxidation as well as iron oxide and titanium oxide phases of a core-shell morphology for charge transfer and oxygen reduction, denoted as CFT. The different surface energies of 0.78 (anatase) and 0.93 J m-2 (rutile) for titanium oxide and 1.39 J m-2 for iron oxide result in a core-shell morphology. The band gaps for iron oxide (2.02 eV), titanium oxide (≈3 eV), and cobalt hydroxide carbonate (3.80 eV) sites reveal that the CFT photocatalyst allows visible-to-UV light absorption. The 18O2 isotope-labeling experiments prove that the core-shell structure promotes hole transfer toward the water oxidation site. Additionally, the hole-induced H2O2 decomposition at the oxygen reduction site is efficiently hindered. Moreover, the photogenerated electrons transfer toward the oxygen reduction site to produce H2O2 from O2 with ≈10-fold higher activity than those by conventional single- or dual-phase photocatalysts, while giving robust cycle stability.
中文翻译:

三相金属氧化物光催化剂用于从氧还原和水氧化反应位点特异性生产过氧化氢
寻找能够将分子氧高活性、选择性和稳定地转化为过氧化氢的光催化剂引起了全世界的兴趣。在这里,作者报告了通过具有用于水氧化的氢氧化钴碳酸盐纳米片相以及氧化铁和氧化钛相的三相金属氧化物光催化剂以≈100% 的选择性和稳定的循环稳定性将 O 2有效转化为 H 2 O 2用于电荷转移和氧还原的核壳形态,表示为CFT。氧化钛的不同表面能分别为 0.78(锐钛矿)和 0.93 J m -2(金红石)和 1.39 J m -2氧化铁导致核壳形态。氧化铁 (2.02 eV)、二氧化钛 (≈3 eV) 和氢氧化钴 (3.80 eV) 位点的带隙表明,CFT 光催化剂允许可见光至紫外光吸收。18 O 2同位素标记实验证明核壳结构促进空穴向水氧化位点转移。另外,有效地阻碍了氧还原位点处的空穴诱导的H 2 O 2分解。此外,光生电子向氧还原位点转移,从O 2产生H 2 O 2与传统的单相或双相光催化剂相比,活性高约 10 倍,同时具有强大的循环稳定性。
更新日期:2022-02-25
中文翻译:

三相金属氧化物光催化剂用于从氧还原和水氧化反应位点特异性生产过氧化氢
寻找能够将分子氧高活性、选择性和稳定地转化为过氧化氢的光催化剂引起了全世界的兴趣。在这里,作者报告了通过具有用于水氧化的氢氧化钴碳酸盐纳米片相以及氧化铁和氧化钛相的三相金属氧化物光催化剂以≈100% 的选择性和稳定的循环稳定性将 O 2有效转化为 H 2 O 2用于电荷转移和氧还原的核壳形态,表示为CFT。氧化钛的不同表面能分别为 0.78(锐钛矿)和 0.93 J m -2(金红石)和 1.39 J m -2氧化铁导致核壳形态。氧化铁 (2.02 eV)、二氧化钛 (≈3 eV) 和氢氧化钴 (3.80 eV) 位点的带隙表明,CFT 光催化剂允许可见光至紫外光吸收。18 O 2同位素标记实验证明核壳结构促进空穴向水氧化位点转移。另外,有效地阻碍了氧还原位点处的空穴诱导的H 2 O 2分解。此外,光生电子向氧还原位点转移,从O 2产生H 2 O 2与传统的单相或双相光催化剂相比,活性高约 10 倍,同时具有强大的循环稳定性。































 京公网安备 11010802027423号
京公网安备 11010802027423号