当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First-Principles Surface Characterization and Water Adsorption of Fe3P Schreibersite
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2022-02-24 , DOI: 10.1021/acsearthspacechem.1c00399
Riccardo Dettori 1 , Nir Goldman 1, 2
ACS Earth and Space Chemistry ( IF 2.9 ) Pub Date : 2022-02-24 , DOI: 10.1021/acsearthspacechem.1c00399
Riccardo Dettori 1 , Nir Goldman 1, 2
Affiliation
|
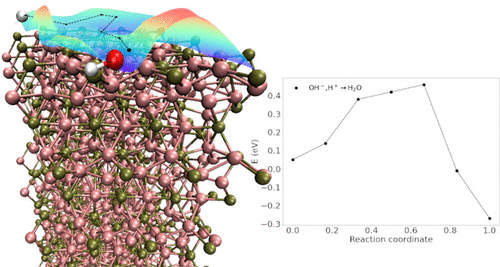
The meteoritic mineral schreibersite, e.g., Fe3P, is a proposed abiotic source of phosphorus for phosphate ion (PO4–) production, needed for nucleobases, phospholipids, and other life building materials. Schreibersite could have acted as both a source of elemental phosphorus and as a catalyst, and the hostile conditions on early Earth could have accelerated its degradation in different environments. Here, we present results from quantum calculations of bulk schreibersite and of its low Miller index surfaces. We also investigate water surface adsorption and identify possible dissociation pathways on the most stable facet. Our calculations provide useful chemical insights into schreibersite interactions in aqueous environments, paving the way for further detailed investigation on more reactive surfaces. Our results help provide a “bottom-up” understanding for phosphorylated organic synthesis on the primitive planet and its role in producing life building molecules.
中文翻译:

Fe3P Schreibersite 的第一原理表面表征和水吸附
陨石矿物 schreibersite,例如 Fe 3 P,是磷酸盐离子(PO 4 -) 生产,需要核碱基、磷脂和其他生命构建材料。Schreibersite 可以作为元素磷的来源和催化剂,早期地球上的恶劣条件可能加速了它在不同环境中的降解。在这里,我们展示了大块 schreibersite 及其低米勒指数表面的量子计算结果。我们还研究了水面吸附并确定了最稳定面上可能的解离途径。我们的计算为水环境中的施莱伯斯矿相互作用提供了有用的化学见解,为进一步详细研究更具反应性的表面铺平了道路。
更新日期:2022-02-24
中文翻译:

Fe3P Schreibersite 的第一原理表面表征和水吸附
陨石矿物 schreibersite,例如 Fe 3 P,是磷酸盐离子(PO 4 -) 生产,需要核碱基、磷脂和其他生命构建材料。Schreibersite 可以作为元素磷的来源和催化剂,早期地球上的恶劣条件可能加速了它在不同环境中的降解。在这里,我们展示了大块 schreibersite 及其低米勒指数表面的量子计算结果。我们还研究了水面吸附并确定了最稳定面上可能的解离途径。我们的计算为水环境中的施莱伯斯矿相互作用提供了有用的化学见解,为进一步详细研究更具反应性的表面铺平了道路。






































 京公网安备 11010802027423号
京公网安备 11010802027423号