当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure–Activity Relationship Study of Indolin-5-yl-cyclopropanamine Derivatives as Selective Lysine Specific Demethylase 1 (LSD1) Inhibitors
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-02-24 , DOI: 10.1021/acs.jmedchem.1c02156 Chunpu Li 1, 2, 3 , Mingbo Su 4 , Wei Zhu 1 , Weijuan Kan 1 , Tianpeng Ge 1, 5 , Gaoya Xu 1 , Shuni Wang 1 , Li Sheng 1 , Feng Gao 1 , Yunfei Ye 6 , Jiang Wang 1, 2 , Yubo Zhou 1, 3, 4, 7 , Jia Li 1, 2, 3, 4, 7 , Hong Liu 1, 2, 3, 4
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-02-24 , DOI: 10.1021/acs.jmedchem.1c02156 Chunpu Li 1, 2, 3 , Mingbo Su 4 , Wei Zhu 1 , Weijuan Kan 1 , Tianpeng Ge 1, 5 , Gaoya Xu 1 , Shuni Wang 1 , Li Sheng 1 , Feng Gao 1 , Yunfei Ye 6 , Jiang Wang 1, 2 , Yubo Zhou 1, 3, 4, 7 , Jia Li 1, 2, 3, 4, 7 , Hong Liu 1, 2, 3, 4
Affiliation
|
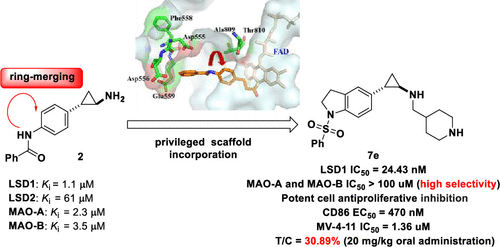
LSD1 is identified as an essential drug target, which is closely correlated to the development of several tumor types. In this work, on the basis of comprehensive analysis of the binding site of LSD1 and other FAD-dependent enzymes, a novel series of potent and selective LSD1 inhibitors were designed by incorporation of privileged indoline scaffold strategies. Representative compound 7e (LSD1; IC50 = 24.43 nM, selectivity over LSD2 and MAOs of >200- and 4000-fold) possessed selective antiproliferative activities against MV-4-11 cell lines. Further study indicates that 7e could activate CD86 expression (EC50 = 470 nM) and induce differentiation of AML cell lines. More importantly, compound 7e demonstrated an acceptable oral PK profile and good in vivo antitumor efficacy with a T/C value of 30.89% in an MV-4-11 xenograft mouse model. Collectively, this work provides a promising lead compound for the development of novel LSD1 inhibitors for the treatment of AML.
中文翻译:

Indolin-5-yl-cyclopropanamine 衍生物作为选择性赖氨酸特异性脱甲基酶 1 (LSD1) 抑制剂的构效关系研究
LSD1 被确定为一种重要的药物靶点,它与几种肿瘤类型的发展密切相关。在这项工作中,在综合分析 LSD1 和其他 FAD 依赖性酶的结合位点的基础上,通过结合特权二氢吲哚支架策略,设计了一系列新的强效和选择性 LSD1 抑制剂。代表性化合物7e(LSD1;IC 50 = 24.43 nM,对 LSD2 的选择性和大于 200 倍和 4000 倍的 MAO)对 MV-4-11 细胞系具有选择性抗增殖活性。进一步研究表明,7e可以激活 CD86 表达 (EC 50 = 470 nM) 并诱导 AML 细胞系的分化。更重要的是,化合物7e在 MV-4-11 异种移植小鼠模型中证明了可接受的口服 PK 曲线和良好的体内抗肿瘤功效,T / C值为 30.89%。总的来说,这项工作为开发用于治疗 AML 的新型 LSD1 抑制剂提供了一种有前景的先导化合物。
更新日期:2022-02-24
中文翻译:

Indolin-5-yl-cyclopropanamine 衍生物作为选择性赖氨酸特异性脱甲基酶 1 (LSD1) 抑制剂的构效关系研究
LSD1 被确定为一种重要的药物靶点,它与几种肿瘤类型的发展密切相关。在这项工作中,在综合分析 LSD1 和其他 FAD 依赖性酶的结合位点的基础上,通过结合特权二氢吲哚支架策略,设计了一系列新的强效和选择性 LSD1 抑制剂。代表性化合物7e(LSD1;IC 50 = 24.43 nM,对 LSD2 的选择性和大于 200 倍和 4000 倍的 MAO)对 MV-4-11 细胞系具有选择性抗增殖活性。进一步研究表明,7e可以激活 CD86 表达 (EC 50 = 470 nM) 并诱导 AML 细胞系的分化。更重要的是,化合物7e在 MV-4-11 异种移植小鼠模型中证明了可接受的口服 PK 曲线和良好的体内抗肿瘤功效,T / C值为 30.89%。总的来说,这项工作为开发用于治疗 AML 的新型 LSD1 抑制剂提供了一种有前景的先导化合物。































 京公网安备 11010802027423号
京公网安备 11010802027423号