当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New multiferroic BiFeO3 with large polarization
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-02-15 , DOI: 10.1039/d1cp05452j Runqing Zhang 1 , Peiju Hu 1 , Lingling Bai 1 , Xing Xie 1 , Huafeng Dong 1, 2 , Minru Wen 1 , Zhongfei Mu 3 , Xin Zhang 1 , Fugen Wu 4
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2022-02-15 , DOI: 10.1039/d1cp05452j Runqing Zhang 1 , Peiju Hu 1 , Lingling Bai 1 , Xing Xie 1 , Huafeng Dong 1, 2 , Minru Wen 1 , Zhongfei Mu 3 , Xin Zhang 1 , Fugen Wu 4
Affiliation
|
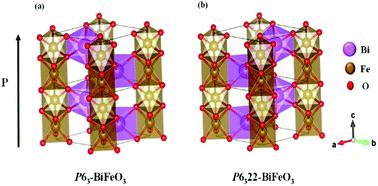
BiFeO3 is one of the most widely studied multiferroic materials, because of its large spontaneous polarization at room temperature, as well as ferroelasticity and antiferromagnetism. Using an ab initio evolutionary algorithm, we found two new dynamically stable BiFeO3 structures (P63 and P6322) at ambient pressure. Their energy is only 0.0662 and 0.0659 eV per atom higher than the famous R3c–BiFeO3, and they have large spontaneous polarization, i.e., 71.82 μC cm−2 and 86.06 μC cm−2, respectively. The spontaneous polarization is caused by the movement of the Bi3+ atom along the [001] direction and mainly comes from the 6s electron of Bi3+. Interestingly, there is no lone pair electron of Bi3+, which is different from R3c–BiFeO3. The new structures have the same magnetic configurations as R3c–BiFeO3 (G-type antiferromagnetism), but they are characterized by one-dimensional channels linked by a group of two via surface-sharing oxygen octahedra. Due to the similarity of the two structures, both of them have indirect bandgap structures, and the bandgaps are 2.62 eV and 2.60 eV, respectively. This work not only broadens the structural diversity of BiFeO3 but also has constructive significance for the study of spontaneous polarization of new structures of multiferroic materials.
中文翻译:

具有大极化的新型多铁性 BiFeO3
BiFeO 3是研究最广泛的多铁性材料之一,因为它在室温下具有较大的自发极化,以及铁弹性和反铁磁性。使用从头算进化算法,我们在环境压力下发现了两个新的动态稳定的 BiFeO 3结构(P 6 3和P 6 3 22)。它们的能量仅比著名的R 3 c -BiFeO 3高0.0662和0.0659 eV/原子,并且它们具有较大的自发极化,即71.82 μC cm -2和86.06 μC cm -2, 分别。自发极化是由Bi 3+原子沿[001]方向运动引起的,主要来自Bi 3+的6s电子。有趣的是,Bi 3+没有孤对电子,这与R 3 c -BiFeO 3不同。新结构具有与R 3 c –BiFeO 3 (G型反铁磁性)相同的磁性结构,但它们的特点是由一组两个通孔连接的一维通道表面共享氧八面体。由于两种结构的相似性,它们都具有间接带隙结构,带隙分别为2.62 eV和2.60 eV。该工作不仅拓宽了BiFeO 3的结构多样性,而且对研究多铁性材料新结构的自发极化具有建设性意义。
更新日期:2022-02-15
中文翻译:

具有大极化的新型多铁性 BiFeO3
BiFeO 3是研究最广泛的多铁性材料之一,因为它在室温下具有较大的自发极化,以及铁弹性和反铁磁性。使用从头算进化算法,我们在环境压力下发现了两个新的动态稳定的 BiFeO 3结构(P 6 3和P 6 3 22)。它们的能量仅比著名的R 3 c -BiFeO 3高0.0662和0.0659 eV/原子,并且它们具有较大的自发极化,即71.82 μC cm -2和86.06 μC cm -2, 分别。自发极化是由Bi 3+原子沿[001]方向运动引起的,主要来自Bi 3+的6s电子。有趣的是,Bi 3+没有孤对电子,这与R 3 c -BiFeO 3不同。新结构具有与R 3 c –BiFeO 3 (G型反铁磁性)相同的磁性结构,但它们的特点是由一组两个通孔连接的一维通道表面共享氧八面体。由于两种结构的相似性,它们都具有间接带隙结构,带隙分别为2.62 eV和2.60 eV。该工作不仅拓宽了BiFeO 3的结构多样性,而且对研究多铁性材料新结构的自发极化具有建设性意义。































 京公网安备 11010802027423号
京公网安备 11010802027423号