当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-free synthesis of gem-difluorinated heterocycles from enaminones and difluorocarbene precursors
Chemical Communications ( IF 4.3 ) Pub Date : 2022-02-14 , DOI: 10.1039/d2cc00383j Fei Wang 1 , Rui Fu 1 , Jie Chen 1, 2 , Jiaxin Rong 1 , Enfu Wang 2 , Jian Zhang 2 , Zhengyu Zhang 1 , Yaojia Jiang 1, 2
Chemical Communications ( IF 4.3 ) Pub Date : 2022-02-14 , DOI: 10.1039/d2cc00383j Fei Wang 1 , Rui Fu 1 , Jie Chen 1, 2 , Jiaxin Rong 1 , Enfu Wang 2 , Jian Zhang 2 , Zhengyu Zhang 1 , Yaojia Jiang 1, 2
Affiliation
|
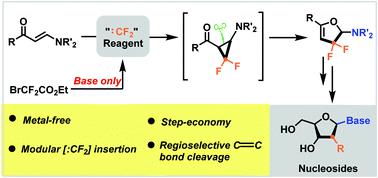
A cascade strategy to synthesise gem-difluorinated 2H-furans from reactions of BrCF2CO2Et with enaminones has been described. The reactions tolerate a wide variety of functional groups under metal-free conditions. An active aminocyclopropane is proposed to be a key intermediate through the cyclopropanation of difluorocarbene with enaminones, which further triggers a regioselective C–C bond cleavage in situ to afford the corresponding gem-difluorinated 2H-furans.
中文翻译:

从烯胺酮和二氟卡宾前体无金属合成偕二氟化杂环
已经描述了由 BrCF 2 CO 2 Et 与烯胺酮反应合成偕二氟化 2 H-呋喃的级联策略。该反应在无金属条件下耐受多种官能团。通过二氟卡宾与烯胺酮的环丙烷化反应,活性氨基环丙烷被认为是关键中间体,这进一步引发了区域选择性 C-C 键原位断裂,得到相应的偕二氟化2 H-呋喃。
更新日期:2022-02-14
中文翻译:

从烯胺酮和二氟卡宾前体无金属合成偕二氟化杂环
已经描述了由 BrCF 2 CO 2 Et 与烯胺酮反应合成偕二氟化 2 H-呋喃的级联策略。该反应在无金属条件下耐受多种官能团。通过二氟卡宾与烯胺酮的环丙烷化反应,活性氨基环丙烷被认为是关键中间体,这进一步引发了区域选择性 C-C 键原位断裂,得到相应的偕二氟化2 H-呋喃。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号