Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cleavage of Abasic Sites in DNA by an Aminoquinoxaline Compound: Augmented Cytotoxicity and DNA Damage in Combination with an Anticancer Drug Chlorambucil in Human Colorectal Carcinoma Cells
ACS Omega ( IF 3.7 ) Pub Date : 2022-02-18 , DOI: 10.1021/acsomega.1c04962 Chandra Sova Mandi 1 , Tridib Mahata 1 , Dipendu Patra 1, 2 , Jeet Chakraborty 1 , Achyut Bora 1, 2 , Ritesh Pal 1, 2 , Sanjay Dutta 1, 2
ACS Omega ( IF 3.7 ) Pub Date : 2022-02-18 , DOI: 10.1021/acsomega.1c04962 Chandra Sova Mandi 1 , Tridib Mahata 1 , Dipendu Patra 1, 2 , Jeet Chakraborty 1 , Achyut Bora 1, 2 , Ritesh Pal 1, 2 , Sanjay Dutta 1, 2
Affiliation
|
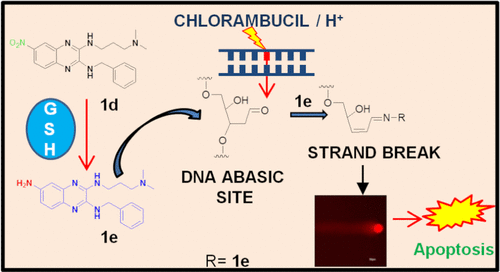
The elevated level of endogenous oxidative DNA damage and spontaneous deamination of DNA bases in cancer cells substantially increase the abasic sites in DNA via base excision repairs (BERs). Thus, the predominant BER pathway is a favorable target for cancer therapy. Interestingly, elevated levels of glutathione (GSH) in certain cancer cells, such as colon cancer, are associated with acquired resistance to several chemotherapeutic agents, which increase the difficulty for the treatment of cancer. Here, we have reported an ideal nitro group-containing monoquinoxaline DNA intercalator (1d), which is reduced into a fluorescent quinoxaline amine (1e) in the presence of GSH; concurrently, 1e (∼100 nM concentration) selectively causes the in vitro cleavage of abasic sites in DNA. 1e also binds to the tetrahydrofuran analogue of the abasic site in the nanomolar to low micromolar range depending on the nucleotide sequence opposite to the abasic site and also induces a structural change in abasic DNA. Furthermore, the amine compound (1e) augments the response of the specific bifunctional alkylating drug chlorambucil at a much lower concentration in the human colorectal carcinoma cell (HCT-116), and their combination shows a potential strategy for targeted therapy. Alone or in combination, 1d and 1e lead to a cascade of cellular events such as induction of DNA double-stranded breaks and cell arrest at G0/G1 and G2/M phases, eventually leading to apoptotic cell death in HCT-116 cells. Hence, the outcome of this study provides a definitive approach that will help optimize the therapeutic applications for targeting the abasic site in cancer cells.
中文翻译:

氨基喹喔啉化合物对 DNA 中无碱基位点的切割:与抗癌药物苯丁酸氮芥联合在人结直肠癌细胞中增强细胞毒性和 DNA 损伤
癌细胞中内源性氧化性 DNA 损伤水平升高和 DNA 碱基自发脱氨作用通过碱基切除修复 (BER) 显着增加了 DNA 中的无碱基位点。因此,主要的 BER 途径是癌症治疗的有利目标。有趣的是,某些癌细胞(如结肠癌)中谷胱甘肽(GSH)水平升高与对几种化疗药物的获得性耐药有关,这增加了癌症治疗的难度。在这里,我们报道了一种理想的含硝基的单喹喔啉 DNA 嵌入剂 ( 1d ),它在 GSH 存在下被还原成荧光喹喔啉胺 ( 1e );同时,1e(~100 nM 浓度)选择性地导致体外DNA 中脱碱基位点的切割。根据与脱碱基位点相反的核苷酸序列, 1e还与纳摩尔到低微摩尔范围内的脱碱基位点的四氢呋喃类似物结合,并且还诱导脱碱基 DNA 的结构变化。此外,胺化合物 ( 1e ) 在人结肠直肠癌细胞 (HCT-116) 中以低得多的浓度增强了特定双功能烷基化药物苯丁酸氮芥的反应,它们的组合显示了靶向治疗的潜在策略。单独或组合,1d和1e导致一系列细胞事件,例如诱导 DNA 双链断裂和 G 0 /G 1和 G 2细胞停滞/M 期,最终导致 HCT-116 细胞凋亡。因此,本研究的结果提供了一种明确的方法,有助于优化靶向癌细胞中无碱基位点的治疗应用。
更新日期:2022-02-18
中文翻译:

氨基喹喔啉化合物对 DNA 中无碱基位点的切割:与抗癌药物苯丁酸氮芥联合在人结直肠癌细胞中增强细胞毒性和 DNA 损伤
癌细胞中内源性氧化性 DNA 损伤水平升高和 DNA 碱基自发脱氨作用通过碱基切除修复 (BER) 显着增加了 DNA 中的无碱基位点。因此,主要的 BER 途径是癌症治疗的有利目标。有趣的是,某些癌细胞(如结肠癌)中谷胱甘肽(GSH)水平升高与对几种化疗药物的获得性耐药有关,这增加了癌症治疗的难度。在这里,我们报道了一种理想的含硝基的单喹喔啉 DNA 嵌入剂 ( 1d ),它在 GSH 存在下被还原成荧光喹喔啉胺 ( 1e );同时,1e(~100 nM 浓度)选择性地导致体外DNA 中脱碱基位点的切割。根据与脱碱基位点相反的核苷酸序列, 1e还与纳摩尔到低微摩尔范围内的脱碱基位点的四氢呋喃类似物结合,并且还诱导脱碱基 DNA 的结构变化。此外,胺化合物 ( 1e ) 在人结肠直肠癌细胞 (HCT-116) 中以低得多的浓度增强了特定双功能烷基化药物苯丁酸氮芥的反应,它们的组合显示了靶向治疗的潜在策略。单独或组合,1d和1e导致一系列细胞事件,例如诱导 DNA 双链断裂和 G 0 /G 1和 G 2细胞停滞/M 期,最终导致 HCT-116 细胞凋亡。因此,本研究的结果提供了一种明确的方法,有助于优化靶向癌细胞中无碱基位点的治疗应用。












































 京公网安备 11010802027423号
京公网安备 11010802027423号