Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
DFT Modeling of CO2 Adsorption and HCOO• Group Conversion in Anatase Au-TiO2-Based Photocatalysis
ACS Omega ( IF 3.7 ) Pub Date : 2022-02-17 , DOI: 10.1021/acsomega.1c06861
Feitong Wu 1 , Yanping Du 1 , Sijia Lv 1 , Changying Zhao 1, 2 , Xiang Yang 1
ACS Omega ( IF 3.7 ) Pub Date : 2022-02-17 , DOI: 10.1021/acsomega.1c06861
Feitong Wu 1 , Yanping Du 1 , Sijia Lv 1 , Changying Zhao 1, 2 , Xiang Yang 1
Affiliation
|
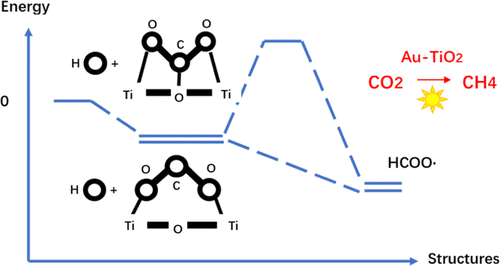
Due to the merits of carbon circulation and hydrocarbon production, solar-assisted photocatalysis has been regarded as an ideal option for securing a sustainable future of energy and environment. In the photocatalytic carbon cycle process, surface reactions including the adsorption of CO2 and the conversion of CO2 into CH4, CH3OH, etc. are crucial to be examined ascribed to their significant influence on the performance of the photocatalysis. Because the conversion reaction starts from the formation of HCOO•, the density functional theory (DFT) model was established in this study to investigate the micromechanism of CO2 adsorption and the conversion of CO2 to HCOO• group in the anatase Au-TiO2 photocatalytic system. The CO2 adsorption bonding in six configurations was simulated, on which basis the effects of the proportion of water molecules and the lattice temperature increase due to the local surface plasmon resonance (LSPR) on the photocatalytic CO2 adsorption and conversion were specifically analyzed. The results show that the experimental conditions that water molecules are released before CO2 are favorable for the formation of the adsorption configuration in which HCOO• tends to be produced without the need of reaction activation energy. This is reasonable since the intermediate C atoms do not participate in bonding under these conditions. Moreover, Au clusters have an insignificant influence on the adsorption behaviors of CO2 including the adsorption sites and configurations on TiO2 surfaces. As a result, the reaction rate is reduced due to the temperature increase caused by the LSPR effect. Nevertheless, the reaction maintains a very high rate. Interestingly, configurations that require activation energy are also possible to be resulted, which exerts a positive influence of temperature on the conversion rate of CO2. It is found that the rate of the reaction can be improved by approximately 1–10 times with a temperature rise of 50 K above the ambient.
中文翻译:

锐钛矿型 Au-TiO2 基光催化中 CO2 吸附和 HCOO• 基团转化的 DFT 建模
由于碳循环和碳氢化合物生产的优点,太阳能辅助光催化已被视为确保能源和环境可持续未来的理想选择。在光催化碳循环过程中,CO 2 的吸附和CO 2 转化为CH 4 、CH 3 OH等的表面反应对光催化性能有显着影响,是研究的关键。由于转化反应是从HCOO •的形成开始的,本研究建立了密度泛函理论(DFT)模型来研究CO 2吸附和CO 2转化的微观机理。HCOO •基团在锐钛矿型Au-TiO 2光催化体系中。模拟了六种构型的CO 2吸附键合,在此基础上具体分析了水分子比例和局部表面等离子共振(LSPR)引起的晶格温度升高对光催化CO 2吸附转化的影响。结果表明,水分子在CO 2之前释放的实验条件有利于HCOO •吸附构型的形成。倾向于在不需要反应活化能的情况下产生。这是合理的,因为在这些条件下中间的 C 原子不参与键合。此外,Au簇对CO 2的吸附行为(包括在TiO 2表面的吸附位点和构型)的影响不显着。结果,由于 LSPR 效应引起的温度升高,反应速率降低。然而,反应保持非常高的速率。有趣的是,也可能产生需要活化能的配置,这对温度对 CO 2的转化率产生积极影响. 发现当温度比环境温度升高 50 K 时,反应速率可以提高大约 1-10 倍。
更新日期:2022-02-17
中文翻译:

锐钛矿型 Au-TiO2 基光催化中 CO2 吸附和 HCOO• 基团转化的 DFT 建模
由于碳循环和碳氢化合物生产的优点,太阳能辅助光催化已被视为确保能源和环境可持续未来的理想选择。在光催化碳循环过程中,CO 2 的吸附和CO 2 转化为CH 4 、CH 3 OH等的表面反应对光催化性能有显着影响,是研究的关键。由于转化反应是从HCOO •的形成开始的,本研究建立了密度泛函理论(DFT)模型来研究CO 2吸附和CO 2转化的微观机理。HCOO •基团在锐钛矿型Au-TiO 2光催化体系中。模拟了六种构型的CO 2吸附键合,在此基础上具体分析了水分子比例和局部表面等离子共振(LSPR)引起的晶格温度升高对光催化CO 2吸附转化的影响。结果表明,水分子在CO 2之前释放的实验条件有利于HCOO •吸附构型的形成。倾向于在不需要反应活化能的情况下产生。这是合理的,因为在这些条件下中间的 C 原子不参与键合。此外,Au簇对CO 2的吸附行为(包括在TiO 2表面的吸附位点和构型)的影响不显着。结果,由于 LSPR 效应引起的温度升高,反应速率降低。然而,反应保持非常高的速率。有趣的是,也可能产生需要活化能的配置,这对温度对 CO 2的转化率产生积极影响. 发现当温度比环境温度升高 50 K 时,反应速率可以提高大约 1-10 倍。

































 京公网安备 11010802027423号
京公网安备 11010802027423号