当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient synthesis of an apremilast precursor and chiral β-hydroxy sulfones via ketoreductase-catalyzed asymmetric reduction
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-02-14 , DOI: 10.1039/d1ob02485j
Jiyang Guo 1 , Xiao Gao 1 , Dong Qian 1 , Huibin Wang 1 , Xian Jia 2 , Wenhe Zhang 1 , Bin Qin 3 , Song You 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2022-02-14 , DOI: 10.1039/d1ob02485j
Jiyang Guo 1 , Xiao Gao 1 , Dong Qian 1 , Huibin Wang 1 , Xian Jia 2 , Wenhe Zhang 1 , Bin Qin 3 , Song You 1
Affiliation
|
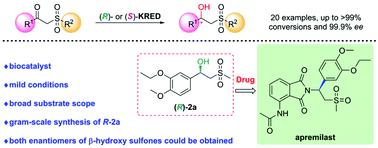
Ketoreductase (KRED)-catalyzed asymmetric reduction of prochiral ketones is an attractive method to synthesize chiral alcohols. Herein, two KREDs LfSDR1-V186A/E141I and CgKR1-F92I with complementary stereopreference were identified towards reduction of apremilast prochiral ketone intermediate 1a. LfSDR1-V186A/E141I exhibited >99% conversion and 99.2% ee yielding an apremilast chiral alcohol intermediate ((R)-2a) at 50 g L−1 substrate loading. Furthermore, we investigated the substrate scope of β-keto sulfones by using LfSDR1-V186A/E141I and CgKR1-F92I to produce both enantiomers of the corresponding β-hydroxy sulfones, with good-to-excellent conversion (up to >99%) and enantioselectivity (up to 99.9% ee) being obtained in most cases. Finally, the gram-scale synthesis of (R)-2a was performed by employing the crude enzyme of LfSDR1-V186A/E141I and BsGDH to afford the desired enantiomer with >99% conversion, 85.9% isolated yield and 99.2% ee. This study presents a biocatalytic strategy to synthesize chiral β-hydroxy sulfones.
中文翻译:

通过酮还原酶催化的不对称还原有效合成阿普斯特前体和手性β-羟基砜
酮还原酶(KRED)催化前手性酮的不对称还原是合成手性醇的一种有吸引力的方法。在此,鉴定出具有互补立体偏好的两个 KRED LfSDR1-V186A/E141I 和 CgKR1-F92I 用于还原阿普斯特前手性酮中间体1a 。 LfSDR1-V186A/E141I表现出>99%转化率和99.2% ee,在50 g L -1底物负载量下产生阿普斯特手性醇中间体(( R ) -2a )。此外,我们通过使用 LfSDR1-V186A/E141I 和 CgKR1-F92I 来研究 β-酮基砜的底物范围,以产生相应 β-羟基砜的两种对映体,具有良好到优异的转化率(高达 >99%)和大多数情况下可获得对映选择性(高达 99.9% ee)。最后,利用LfSDR1-V186A/E141I的粗酶和BsGDH进行克级合成( R ) -2a ,得到所需的对映体,转化率>99%,分离收率85.9%,ee 99.2%。本研究提出了合成手性 β-羟基砜的生物催化策略。
更新日期:2022-02-14
中文翻译:

通过酮还原酶催化的不对称还原有效合成阿普斯特前体和手性β-羟基砜
酮还原酶(KRED)催化前手性酮的不对称还原是合成手性醇的一种有吸引力的方法。在此,鉴定出具有互补立体偏好的两个 KRED LfSDR1-V186A/E141I 和 CgKR1-F92I 用于还原阿普斯特前手性酮中间体1a 。 LfSDR1-V186A/E141I表现出>99%转化率和99.2% ee,在50 g L -1底物负载量下产生阿普斯特手性醇中间体(( R ) -2a )。此外,我们通过使用 LfSDR1-V186A/E141I 和 CgKR1-F92I 来研究 β-酮基砜的底物范围,以产生相应 β-羟基砜的两种对映体,具有良好到优异的转化率(高达 >99%)和大多数情况下可获得对映选择性(高达 99.9% ee)。最后,利用LfSDR1-V186A/E141I的粗酶和BsGDH进行克级合成( R ) -2a ,得到所需的对映体,转化率>99%,分离收率85.9%,ee 99.2%。本研究提出了合成手性 β-羟基砜的生物催化策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号