当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Strategy to Select Macrocyclic Peptides Featuring Asymmetric Molecular Scaffolds as Cyclization Units by Phage Display
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-02-16 , DOI: 10.1021/jacs.1c12822 Titia Rixt Oppewal 1 , Ivar D Jansen 1 , Johan Hekelaar 1 , Clemens Mayer 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-02-16 , DOI: 10.1021/jacs.1c12822 Titia Rixt Oppewal 1 , Ivar D Jansen 1 , Johan Hekelaar 1 , Clemens Mayer 1
Affiliation
|
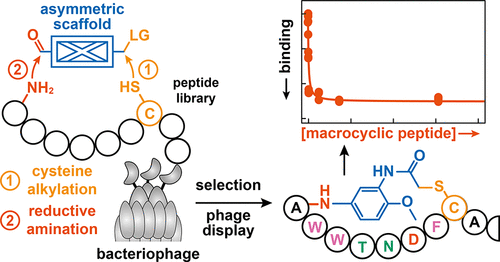
Macrocyclic peptides (MPs) have positioned themselves as a privileged class of compounds for the discovery of therapeutics and development of chemical probes. Aided by the development of powerful selection strategies, high-affinity binders against biomolecular targets can readily be elicited from massive, genetically encoded libraries by affinity selection. For example, in phage display, MPs are accessed on the surface of whole bacteriophages via disulfide formation, the use of (symmetric) crosslinkers, or the incorporation of non-canonical amino acids. To facilitate a straightforward cyclization of linear precursors with asymmetric molecular scaffolds, which are often found at the core of naturally occurring MPs, we report an efficient two-step strategy to access MPs via the programmed modification of a unique cysteine residue and an N-terminal amine. We demonstrate that this approach yields MPs featuring asymmetric cyclization units from both synthetic peptides and when linear precursors are appended onto a phage-coat protein. Finally, we showcase that our cyclization strategy is compatible with traditional phage-display protocols and enables the selection of MP binders against a model target protein from naïve libraries. By enabling the incorporation of non-peptidic moieties that (1) can serve as cyclization units, (2) provide interactions for binding, and/or (3) tailor pharmacological properties, our head-to-side-chain cyclization strategy provides access to a currently under-explored chemical space for the development of chemical probes and therapeutics.
中文翻译:

通过噬菌体展示选择以不对称分子支架为环化单元的大环肽的策略
大环肽 (MP) 已将自己定位为一类特殊化合物,用于发现治疗方法和开发化学探针。在强大的选择策略的开发的帮助下,通过亲和力选择可以很容易地从大量的基因编码文库中引出针对生物分子靶标的高亲和力结合物。例如,在噬菌体展示中,通过二硫键的形成、使用(对称)交联剂或掺入非规范氨基酸,在整个噬菌体的表面上获取 MP。为了促进线性前体与不对称分子支架(通常存在于天然存在的 MP 的核心)的直接环化,我们报告了一种有效的两步策略,通过对独特的半胱氨酸残基和 N 端进行编程修饰来获取 MP胺。我们证明,当线性前体附加到噬菌体外壳蛋白上时,这种方法可以产生具有来自合成肽的不对称环化单元的MP。最后,我们展示了我们的环化策略与传统的噬菌体展示方案兼容,并且能够针对来自初始文库的模型目标蛋白选择 MP 结合物。通过掺入非肽部分(1)可以作为环化单元,(2)提供结合相互作用,和/或(3)定制药理学特性,我们的头到侧链环化策略提供了目前尚未开发化学探针和治疗药物的化学空间。
更新日期:2022-02-16
中文翻译:

通过噬菌体展示选择以不对称分子支架为环化单元的大环肽的策略
大环肽 (MP) 已将自己定位为一类特殊化合物,用于发现治疗方法和开发化学探针。在强大的选择策略的开发的帮助下,通过亲和力选择可以很容易地从大量的基因编码文库中引出针对生物分子靶标的高亲和力结合物。例如,在噬菌体展示中,通过二硫键的形成、使用(对称)交联剂或掺入非规范氨基酸,在整个噬菌体的表面上获取 MP。为了促进线性前体与不对称分子支架(通常存在于天然存在的 MP 的核心)的直接环化,我们报告了一种有效的两步策略,通过对独特的半胱氨酸残基和 N 端进行编程修饰来获取 MP胺。我们证明,当线性前体附加到噬菌体外壳蛋白上时,这种方法可以产生具有来自合成肽的不对称环化单元的MP。最后,我们展示了我们的环化策略与传统的噬菌体展示方案兼容,并且能够针对来自初始文库的模型目标蛋白选择 MP 结合物。通过掺入非肽部分(1)可以作为环化单元,(2)提供结合相互作用,和/或(3)定制药理学特性,我们的头到侧链环化策略提供了目前尚未开发化学探针和治疗药物的化学空间。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号