当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Preclinical Characterization of LY3154885, a Human Dopamine D1 Receptor Positive Allosteric Modulator with an Improved Nonclinical Drug–Drug Interaction Risk Profile
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-02-17 , DOI: 10.1021/acs.jmedchem.1c01887 Junliang Hao 1 , James Beck 1 , Xin Zhou 2 , Gregory L. Lackner 1 , Richard Johnston 1 , Matt Reinhard 1 , Paul Goldsmith 1 , Sean Hollinshead 1 , Veronique Dehlinger 1 , Sandra A. Filla 1 , Xu-shan Wang 3 , Jeffery Richardson 1 , Maria Posada 2 , Mike Mohutsky 2 , Doug Schober 4 , Jason S. Katner 4 , Qi Chen 1 , Bingjie Hu 2 , David M. Remick 5 , David A. Coates 5 , Brian M. Mathes 5 , Mai K. Hawk 5 , Kjell A. Svensson 4 , Erik Hembre 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2022-02-17 , DOI: 10.1021/acs.jmedchem.1c01887 Junliang Hao 1 , James Beck 1 , Xin Zhou 2 , Gregory L. Lackner 1 , Richard Johnston 1 , Matt Reinhard 1 , Paul Goldsmith 1 , Sean Hollinshead 1 , Veronique Dehlinger 1 , Sandra A. Filla 1 , Xu-shan Wang 3 , Jeffery Richardson 1 , Maria Posada 2 , Mike Mohutsky 2 , Doug Schober 4 , Jason S. Katner 4 , Qi Chen 1 , Bingjie Hu 2 , David M. Remick 5 , David A. Coates 5 , Brian M. Mathes 5 , Mai K. Hawk 5 , Kjell A. Svensson 4 , Erik Hembre 1
Affiliation
|
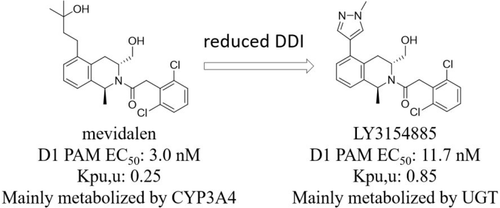
Results from recently completed clinical studies suggest the dopamine D1 receptor positive allosteric modulator (PAM) mevidalen (1) could offer unique value for lewy body dementia (LBD) patients. In nonclinical assessments, 1 was mainly eliminated by CYP3A4-mediated metabolism, therefore at the risk of being a victim of drug–drug interactions (DDI) with CYP3A4 inhibitors and inducers. An effort was initiated to identify a new D1 PAM with an improved DDI risk profile. While attempts to introduce additional metabolic pathways mediated by other CYP isoforms failed to provide molecules with an acceptable profile, we discovered that the relative contribution of CYP-mediated oxidation and UGT-mediated conjugation could be tuned to reduce the CYP3A4-mediated victim DDI risk. We have identified LY3154885 (5), a D1 PAM that possesses similar in vitro and in vivo pharmacologic properties as 1, but is metabolized mainly by UGT, predicting it could potentially offer lower victim DDI risk in clinic.
中文翻译:

LY3154885 的合成和临床前表征,一种人多巴胺 D1 受体正变构调节剂,具有改善的非临床药物-药物相互作用风险状况
最近完成的临床研究结果表明,多巴胺 D1 受体阳性变构调节剂 (PAM) mevidalen ( 1 ) 可为路易体痴呆 (LBD) 患者提供独特的价值。在非临床评估中,1主要通过 CYP3A4 介导的代谢消除,因此有可能成为 CYP3A4 抑制剂和诱导剂的药物相互作用 (DDI) 的受害者。已开始努力确定具有改进的 DDI 风险概况的新 D1 PAM。虽然尝试引入由其他 CYP 同种型介导的额外代谢途径未能为分子提供可接受的特征,但我们发现 CYP 介导的氧化和 UGT 介导的缀合的相对贡献可以调整以降低 CYP3A4 介导的受害者 DDI 风险。我们已经鉴定出 LY3154885 ( 5 ),一种 D1 PAM,具有与1相似的体外和体内药理特性,但主要通过 UGT 代谢,预测它可能在临床上提供较低的受害者 DDI 风险。
更新日期:2022-02-17
中文翻译:

LY3154885 的合成和临床前表征,一种人多巴胺 D1 受体正变构调节剂,具有改善的非临床药物-药物相互作用风险状况
最近完成的临床研究结果表明,多巴胺 D1 受体阳性变构调节剂 (PAM) mevidalen ( 1 ) 可为路易体痴呆 (LBD) 患者提供独特的价值。在非临床评估中,1主要通过 CYP3A4 介导的代谢消除,因此有可能成为 CYP3A4 抑制剂和诱导剂的药物相互作用 (DDI) 的受害者。已开始努力确定具有改进的 DDI 风险概况的新 D1 PAM。虽然尝试引入由其他 CYP 同种型介导的额外代谢途径未能为分子提供可接受的特征,但我们发现 CYP 介导的氧化和 UGT 介导的缀合的相对贡献可以调整以降低 CYP3A4 介导的受害者 DDI 风险。我们已经鉴定出 LY3154885 ( 5 ),一种 D1 PAM,具有与1相似的体外和体内药理特性,但主要通过 UGT 代谢,预测它可能在临床上提供较低的受害者 DDI 风险。






























 京公网安备 11010802027423号
京公网安备 11010802027423号