当前位置:
X-MOL 学术
›
Energy Fuels
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Sulfite Oxidation in NO2 Absorption in a pH-Neutral Scrubber Solution
Energy & Fuels ( IF 5.2 ) Pub Date : 2022-02-17 , DOI: 10.1021/acs.energyfuels.1c03914 Daniel Schmid 1 , Oskar Karlström 1 , Virva Kuvaja 2 , Ilari Vuorinen 2 , Mikko Paavola 2 , Mikko Hupa 1
Energy & Fuels ( IF 5.2 ) Pub Date : 2022-02-17 , DOI: 10.1021/acs.energyfuels.1c03914 Daniel Schmid 1 , Oskar Karlström 1 , Virva Kuvaja 2 , Ilari Vuorinen 2 , Mikko Paavola 2 , Mikko Hupa 1
Affiliation
|
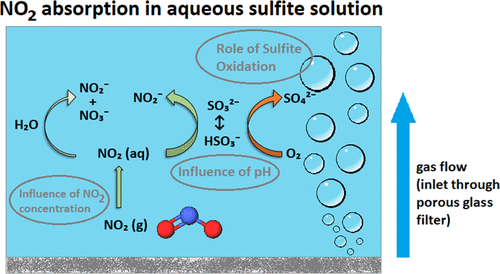
Nitrogen dioxide (NO2) absorption in aqueous sulfite solutions has recently gained more attention to reduce NOx emissions. The NO2 removal efficiency is strongly influenced by the competing reactions of NO2 absorption and sulfite oxidation. The present study investigates the role of sulfite oxidation in NO2 absorption at neutral pH. The effects of sulfite concentration, thiosulfate, pH, NO2 inlet concentration, oxygen concentration on NO2 absorption efficiency, and sulfite oxidation rates were investigated under well-controlled conditions at 25 °C in a laboratory-scale wet scrubbing system. NO2 absorption rates showed a strong dependence on the sulfite concentration and increased linearly with increasing NO2 inlet concentrations between 25 and 150 ppm. At the highest investigated sulfite concentration, 20 mM, the NO2 removal efficiency was around 80%. Oxidation rates of sulfite during NO2 absorption in the presence of 5 vol % O2 increased with increasing sulfite concentrations in the scrubbing solution and ranged from 20 mg·L–1·min–1 with 1 mM sulfite to 200 mg·L–1·min–1 with 20 mM sulfite. The oxidation rate decreased significantly when thiosulfate was added, while the oxidation rates were independent of the oxygen concentration between 2–10 vol % O2. The NO2 absorption rate decreased with decreasing pH between pH 6 and 8 proportionally to the sulfite concentration as predicted by the sulfite–bisulfite equilibrium. The implication from these results is that significantly less sulfite feeding is needed in industrial NO2 absorption if the pH can be maintained at neutral pH, instead of lower pH such as in simultaneous SO2 and NO2 scrubbing.
中文翻译:

亚硫酸盐氧化在 pH 中性洗涤器溶液中吸收 NO2 中的作用
亚硫酸水溶液中的二氧化氮 (NO 2 ) 吸收最近获得了更多关注,以减少 NO x排放。NO 2去除效率受NO 2吸收和亚硫酸盐氧化的竞争反应的强烈影响。本研究调查了亚硫酸盐氧化在中性 pH条件下对 NO 2吸收的作用。在实验室规模的湿式洗涤系统中,在 25 °C 的良好控制条件下,研究了亚硫酸盐浓度、硫代硫酸盐、pH、NO 2入口浓度、氧气浓度对 NO 2吸收效率和亚硫酸盐氧化速率的影响。没有2吸收率表现出对亚硫酸盐浓度的强烈依赖性,并且随着 NO 2入口浓度在 25 和 150 ppm 之间的增加而线性增加。在研究的最高亚硫酸盐浓度 20 mM 下,NO 2去除效率约为 80%。在 5 vol% O 2存在下, NO 2吸收过程中亚硫酸盐的氧化率随着洗涤溶液中亚硫酸盐浓度的增加而增加,范围从 1 mM 亚硫酸盐的 20 mg·L –1 ·min –1到 200 mg·L –1 ·min –1含 20 mM 亚硫酸盐。添加硫代硫酸盐后氧化速率显着降低,而氧化速率与 2-10 vol % O 2之间的氧浓度无关。如亚硫酸盐-亚硫酸氢盐平衡所预测的,NO 2吸收率随着 pH 值在 6 和 8 之间的降低与亚硫酸盐浓度成正比而降低。这些结果的含义是,如果 pH 值可以保持在中性 pH 值,而不是像同时进行 SO 2和 NO 2洗涤的较低 pH 值,则工业 NO 2吸收中需要的亚硫酸盐进料量显着减少。
更新日期:2022-02-17
中文翻译:

亚硫酸盐氧化在 pH 中性洗涤器溶液中吸收 NO2 中的作用
亚硫酸水溶液中的二氧化氮 (NO 2 ) 吸收最近获得了更多关注,以减少 NO x排放。NO 2去除效率受NO 2吸收和亚硫酸盐氧化的竞争反应的强烈影响。本研究调查了亚硫酸盐氧化在中性 pH条件下对 NO 2吸收的作用。在实验室规模的湿式洗涤系统中,在 25 °C 的良好控制条件下,研究了亚硫酸盐浓度、硫代硫酸盐、pH、NO 2入口浓度、氧气浓度对 NO 2吸收效率和亚硫酸盐氧化速率的影响。没有2吸收率表现出对亚硫酸盐浓度的强烈依赖性,并且随着 NO 2入口浓度在 25 和 150 ppm 之间的增加而线性增加。在研究的最高亚硫酸盐浓度 20 mM 下,NO 2去除效率约为 80%。在 5 vol% O 2存在下, NO 2吸收过程中亚硫酸盐的氧化率随着洗涤溶液中亚硫酸盐浓度的增加而增加,范围从 1 mM 亚硫酸盐的 20 mg·L –1 ·min –1到 200 mg·L –1 ·min –1含 20 mM 亚硫酸盐。添加硫代硫酸盐后氧化速率显着降低,而氧化速率与 2-10 vol % O 2之间的氧浓度无关。如亚硫酸盐-亚硫酸氢盐平衡所预测的,NO 2吸收率随着 pH 值在 6 和 8 之间的降低与亚硫酸盐浓度成正比而降低。这些结果的含义是,如果 pH 值可以保持在中性 pH 值,而不是像同时进行 SO 2和 NO 2洗涤的较低 pH 值,则工业 NO 2吸收中需要的亚硫酸盐进料量显着减少。































 京公网安备 11010802027423号
京公网安备 11010802027423号