当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoinduced Annulation of N-Phenylbenzamides for the Synthesis of Phenanthridin-6(5H)-Ones
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-02-15 , DOI: 10.1002/adsc.202101389 Nana Wang 1 , Ding Wang 1 , Yun He 2 , Jin Xi 1 , Tao Wang 1 , Yong Liang 3 , Zunting Zhang 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2022-02-15 , DOI: 10.1002/adsc.202101389 Nana Wang 1 , Ding Wang 1 , Yun He 2 , Jin Xi 1 , Tao Wang 1 , Yong Liang 3 , Zunting Zhang 1
Affiliation
|
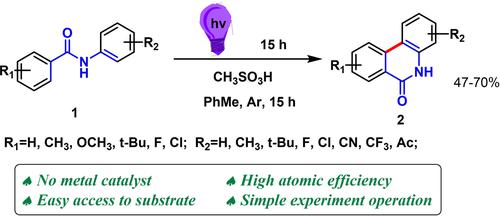
A general concise method for the synthesis phenanthridin-6(5H)-ones via photoinduced intramolecular annulation of N-phenylbenzamides was developed. Under argon atmosphere and room temperature, phenanthridin-6(5H)-ones were obtained via irradiation N-phenylbenzamides with a 280 nm UV lamp in the presence of methanesulfonic acid in toluene. The mechanism is illustrated and believed to proceed in the order of amides tautomerization, 6π-electric cyclization, [1,5]-H shift, amide-imidine tautomerization, keto-enol tautomerism and evolution hydrogen.
中文翻译:

N-苯基苯甲酰胺的光诱导环化合成 Phenothridin-6(5H)-Ones
开发了一种通过光诱导N-苯基苯甲酰胺分子内环化合成菲啶-6(5 H )-ones的通用简明方法。在氩气和室温下,在甲苯中甲磺酸存在下,通过用 280 nm 紫外灯照射N-苯基苯甲酰胺,得到菲啶-6(5 H )-酮。该机理被说明并认为按照酰胺互变异构、6π-电环化、[1,5]-H 位移、酰胺-亚胺互变异构、酮-烯醇互变异构和放氢的顺序进行。
更新日期:2022-02-15
中文翻译:

N-苯基苯甲酰胺的光诱导环化合成 Phenothridin-6(5H)-Ones
开发了一种通过光诱导N-苯基苯甲酰胺分子内环化合成菲啶-6(5 H )-ones的通用简明方法。在氩气和室温下,在甲苯中甲磺酸存在下,通过用 280 nm 紫外灯照射N-苯基苯甲酰胺,得到菲啶-6(5 H )-酮。该机理被说明并认为按照酰胺互变异构、6π-电环化、[1,5]-H 位移、酰胺-亚胺互变异构、酮-烯醇互变异构和放氢的顺序进行。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号