Chemosphere ( IF 8.1 ) Pub Date : 2022-02-16 , DOI: 10.1016/j.chemosphere.2022.134014 Zhangbin Pan 1 , Zhenqi Du 2 , Junqi Jia 3 , Aiguo Lin 4 , Yongqiang Wang 5 , Wuchang Song 6 , Shaohua Sun 6 , Hongbo Wang 5 , Ruibao Jia 6 , Lian Hou 1
|
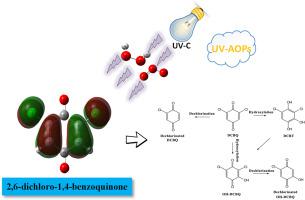
2,6-dichloro-1,4-benzoquinone (DCBQ), a typical representative of Halobenzoquinones, is an emerging aromatic disinfection by-product (DBP) with high toxicity and carcinogenicity, generated commonly through the chlorination in the drinking water disinfection process while there is still a lack of research on its removal. In this study, the effects of ultraviolet-based advanced oxidation processes (UV-AOPs) on the degradation of DCBQ were evaluated. The results showed that UV-AOPs are effective in degrading DCBQ. The removal of DCBQ by UV/H2O2/O3 was more significant than by UV/H2O2 or UV/O3, achieving a 96.7% removal rate at both the O3 and H2O2 doses of 1 mg/L. The results also indicated the alkaline and weakly acidic environments could facilitate the degradation of DCBQ, inorganic anions could inhibit DCBQ degradation and the degree of inhibition increased as the matrix concentration increased. The degradation of DCBQ was inhibited more by the CO32− than the other matrix components, such as Cl− and NO3−. It was shown by the density functional theory simulations and the ultrahigh-performance liquid chromatography (UPLC) - Orbitrap mass spectra that the electrons in DCBQ are mainly on the chlorine atom connected to the carboatomic ring and that OH• can attack the chlorine atom to cause de-chlorination. The DCBQ degradation pathway may involve the oxidation of DCBQ to 3-hydroxy-2,6-DCBQ (HO–DCBQ) and 3,5-dichloro-1,2,4-pyrogallol, the further degradation of intermediate products by OH• to dechlorinated forms of HO–DCBQ and DCBQ.
中文翻译:

UV/H2O2/O3处理降解2,6-二氯-1,4-苯醌:有效性、水基质效应和降解机理
2,6-二氯-1,4-苯醌(DCBQ)是卤代苯醌的典型代表,是一种新兴的芳香族消毒副产物(DBP),具有高毒性和致癌性,通常在饮用水消毒过程中通过氯化作用产生,同时关于它的去除仍然缺乏研究。在这项研究中,评估了基于紫外线的高级氧化工艺 (UV-AOPs) 对 DCBQ 降解的影响。结果表明,UV-AOPs 可有效降解 DCBQ。UV/H 2 O 2 /O 3对 DCBQ 的去除比 UV/H 2 O 2或 UV/O 3更显着, O 3和 H 2的去除率均达到 96.7%O 2剂 1 mg/L。结果还表明碱性和弱酸性环境有利于DCBQ的降解,无机阴离子能抑制DCBQ的降解,且抑制程度随着基质浓度的增加而增加。CO 3 2-对 DCBQ 的降解的抑制作用比其他基质成分(如 Cl -和 NO 3 - )更强。. 密度泛函理论模拟和超高效液相色谱(UPLC)-Orbitrap质谱表明,DCBQ中的电子主要位于与碳原子环相连的氯原子上,OH•可以攻击氯原子引起脱氯。DCBQ 降解途径可能涉及 DCBQ 氧化为 3-羟基-2,6-DCBQ (HO-DCBQ) 和 3,5-二氯-1,2,4-邻苯三酚,中间产物被 OH• 进一步降解为脱氯形式的 HO-DCBQ 和 DCBQ。






























 京公网安备 11010802027423号
京公网安备 11010802027423号