当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation of Dihydronaphthofurans from α-Hydroxyl Ketones via a One-Pot Multicomponent Reaction Based on Heyns Rearrangement
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-02-15 , DOI: 10.1021/acs.joc.1c02958 Jin Huang 1 , Jin-Fang Chen 2 , Xin Cui 1 , Jin-Zhong Zhao 2 , Zhuo Tang 1 , Guang-Xun Li 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-02-15 , DOI: 10.1021/acs.joc.1c02958 Jin Huang 1 , Jin-Fang Chen 2 , Xin Cui 1 , Jin-Zhong Zhao 2 , Zhuo Tang 1 , Guang-Xun Li 1
Affiliation
|
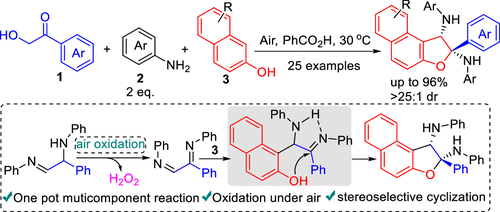
Polysubstituted 1,2-dihydronaphthofurans were efficiently obtained in high yields and good diastereoselectivities with readily available substrates. The reaction proceeds smoothly via a series of tandem reactions, including Heyns rearrangement, oxidation, Friedel–Crafts reaction, and cyclization. The high stereoselectivity of the reaction is ascribed to the activation of the imine via an intramolecular hydrogen bond. Air is directly used as the oxidation medium, which makes the reaction safe and easy to perform. Moreover, the reaction features multiple components, which ensures the diversity of products.
中文翻译:

基于Heyns重排的一锅多组分反应α-羟基酮制备二氢萘呋喃
多取代的 1,2-二氢萘呋喃以高产率和良好的非对映选择性有效地获得,并且具有易于获得的底物。该反应通过一系列串联反应顺利进行,包括海恩斯重排、氧化、傅克反应和环化反应。该反应的高立体选择性归因于亚胺通过分子内氢键的活化。直接使用空气作为氧化介质,使反应安全且易于进行。此外,该反应具有多种组分,保证了产物的多样性。
更新日期:2022-02-15
中文翻译:

基于Heyns重排的一锅多组分反应α-羟基酮制备二氢萘呋喃
多取代的 1,2-二氢萘呋喃以高产率和良好的非对映选择性有效地获得,并且具有易于获得的底物。该反应通过一系列串联反应顺利进行,包括海恩斯重排、氧化、傅克反应和环化反应。该反应的高立体选择性归因于亚胺通过分子内氢键的活化。直接使用空气作为氧化介质,使反应安全且易于进行。此外,该反应具有多种组分,保证了产物的多样性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号