当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
3-Trifluoroacetyl-quinolin-2(1H)-ones as Carbonyl and Acid Surrogates in the Passerini-/Ugi-Type Reaction
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-02-14 , DOI: 10.1021/acs.joc.1c02107 Desagoni Madhu 1, 2 , Vatsala Rani Jetti 2 , Banda Narsaiah 2 , Nagender Punna 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-02-14 , DOI: 10.1021/acs.joc.1c02107 Desagoni Madhu 1, 2 , Vatsala Rani Jetti 2 , Banda Narsaiah 2 , Nagender Punna 1, 2
Affiliation
|
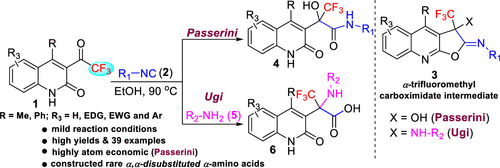
Herein, we report tailored 3-trifluoroacetyl-quinolin-2(1H)-ones (1) as carbonyl and acid surrogates in Passerini- and Ugi-type reactions for the synthesis of α-trifluoromethyl-α-hydroxy carboxamides (4) and α-trifluoromethyl α-amino acids (6) in high yields, respectively. The reaction proceeds under mild reaction conditions via an exocyclic carboximidate intermediate (3). The amide group in compound 1 acts as an acid component as well as a reversible oxygen nucleophile to facilitate the reaction.
中文翻译:

3-Trifluoroacetyl-quinolin-2(1H)-ones 作为 Passerini-/Ugi 型反应中的羰基和酸替代物
在此,我们报告了定制的 3-三氟乙酰基-喹啉-2(1 H )-酮 ( 1 ) 作为 Passerini 和 Ugi 型反应中的羰基和酸替代物,用于合成 α-三氟甲基-α-羟基甲酰胺 ( 4 ) 和α-三氟甲基α-氨基酸 ( 6 ) 的产率分别很高。该反应在温和的反应条件下通过环外羧酸亚胺中间体 ( 3 ) 进行。化合物1中的酰胺基团作为酸组分以及可逆的氧亲核试剂来促进反应。
更新日期:2022-02-14
中文翻译:

3-Trifluoroacetyl-quinolin-2(1H)-ones 作为 Passerini-/Ugi 型反应中的羰基和酸替代物
在此,我们报告了定制的 3-三氟乙酰基-喹啉-2(1 H )-酮 ( 1 ) 作为 Passerini 和 Ugi 型反应中的羰基和酸替代物,用于合成 α-三氟甲基-α-羟基甲酰胺 ( 4 ) 和α-三氟甲基α-氨基酸 ( 6 ) 的产率分别很高。该反应在温和的反应条件下通过环外羧酸亚胺中间体 ( 3 ) 进行。化合物1中的酰胺基团作为酸组分以及可逆的氧亲核试剂来促进反应。







































 京公网安备 11010802027423号
京公网安备 11010802027423号