Toxicology and Applied Pharmacology ( IF 3.3 ) Pub Date : 2022-02-15 , DOI: 10.1016/j.taap.2022.115923 Yin Zhu 1 , Xiaozhi Wang 2 , Lujian Zhu 3 , Yulu Tu 3 , Wanting Chen 4 , Lingwen Gong 4 , Tongtong Pan 3 , Hongwei Lin 3 , Jing Lin 3 , Huiling Sun 5 , Yuli Ge 5 , Li Wei 4 , Yu Guo 4 , Caide Lu 6 , Yongping Chen 3 , Lanman Xu 1
|
Background
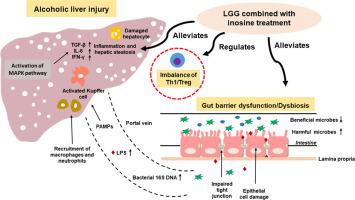
Intestinal epithelial barrier disruption and bacterial translocation exacerbates the progression of alcoholic liver disease. Lactobacillus rhamnosus GG (LGG), a probiotic, has been shown benefits in chronic liver disease and in regulating gut dysbiosis. Previous studies showed the protective roles of LGG in ethanol-disrupted gut barrier functions and liver injury. Inosine, a metabolite produced by intestinal bacteria, has the anti-inflammatory and immunregulatory functions. In this study, the synergistic effect of LGG and inosine was investigated in a mouse model of alcohol-induced liver disease (ALD).
Methods
Male C57BL/6 mice were fed with a Lieber-DeCarli diet containing 5% alcohol for four weeks to establish a model of alcohol-induced liver injury. LGG or a combination of LGG and inosine were administrated orally to explore a new therapeutic method for alcohol-induced liver disease and to investigate the underlying mechanisms. Liver damage was evaluated by transaminases and pathological changes. Tight junction proteins, composition of the gut microbiome, cytokines, lipopolysaccharides (LPS), glutathione (GSH), superoxide dismutase (SOD), malondialdehyde (MDA), F4/80+ macrophages, as well as p38, Jun N-terminal kinase (JNK), were determined by qRT-PCR, RNAseq, ELISA, IHC and western blot. Regulatory T (Treg) cells were characterized by positive staining of CD4, CD25 and Foxp3 using flow cytometry. IFN-γ–producing CD4+ T (Th1) cells were examined by intracellular cytokine staining.
Results
Alcohol consumption induced elevated liver enzymes, steatosis and inflammation, while LGG combined with inosine treatment was more significant to ameliorate these symptoms compared with LGG alone. When LGG combined with inosine were administered to ALD mice, intestinal microecology significantly improved reflected by intestinal villi and tight junction proteins recovery and the restoration of intestinal flora. Combined therapy inhibited phosphorylation of p38 and JNK to alleviate hepatic inflammation. Moreover, flow cytometry analysis showed that long-term excessive alcohol consumption reduced Tregs population while increased Th1 population, which was restored by a combination of LGG and inosine treatment.
Conclusions
The findings from the study indicate that the combined LGG and inosine treatment ameliorates ALD by improving the gut ecosystem, intestinal barrier function, immune homeostasis and liver injury.
中文翻译:

鼠李糖乳杆菌GG联合肌苷通过调节肠屏障和Treg/Th1细胞改善酒精性肝损伤
背景
肠上皮屏障破坏和细菌易位加剧了酒精性肝病的进展。鼠李糖乳杆菌GG (LGG) 是一种益生菌,已被证明对慢性肝病和调节肠道菌群失调有益。先前的研究表明 LGG 在乙醇破坏的肠道屏障功能和肝损伤中的保护作用。肌苷是肠道细菌产生的代谢产物,具有抗炎和免疫调节功能。在这项研究中,在酒精性肝病 (ALD) 的小鼠模型中研究了 LGG 和肌苷的协同作用。
方法
给雄性 C57BL/6 小鼠喂食含 5% 酒精的 Lieber-DeCarli 饮食 4 周,以建立酒精性肝损伤模型。口服 LGG 或 LGG 与肌苷的组合以探索酒精性肝病的新治疗方法并研究其潜在机制。通过转氨酶和病理变化评估肝损伤。紧密连接蛋白、肠道微生物组的组成、细胞因子、脂多糖 (LPS)、谷胱甘肽 (GSH)、超氧化物歧化酶 (SOD)、丙二醛 (MDA)、F4/80+ 巨噬细胞,以及 p38、Jun N-末端激酶 ( JNK),通过 qRT-PCR、RNAseq、ELISA、IHC 和蛋白质印迹测定。调节性 T (Treg) 细胞的特征是使用流式细胞术对 CD4、CD25 和 Foxp3 进行阳性染色。产生 IFN-γ 的 CD4 +通过细胞内细胞因子染色检查 T (Th1) 细胞。
结果
饮酒会导致肝酶升高、脂肪变性和炎症,而与单独使用 LGG 相比,LGG 联合肌苷治疗更能显着改善这些症状。当LGG联合肌苷给予ALD小鼠时,肠道微生态显着改善,表现为肠绒毛和紧密连接蛋白的恢复以及肠道菌群的恢复。联合治疗抑制 p38 和 JNK 的磷酸化以减轻肝脏炎症。此外,流式细胞仪分析表明,长期过量饮酒减少了 Tregs 的数量,同时增加了 Th1 的数量,通过 LGG 和肌苷治疗的结合可以恢复。
结论
研究结果表明,LGG 和肌苷联合治疗可通过改善肠道生态系统、肠道屏障功能、免疫稳态和肝损伤来改善 ALD。

































 京公网安备 11010802027423号
京公网安备 11010802027423号