当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dancing on Ropes - Enantioselective Functionalization of Preformed Four-Membered Carbocycles
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2022-02-12 , DOI: 10.1002/cjoc.202100879
Jun Chen 1 , Qiang Zhou 1 , Huayi Fang 2 , Ping Lu 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2022-02-12 , DOI: 10.1002/cjoc.202100879
Jun Chen 1 , Qiang Zhou 1 , Huayi Fang 2 , Ping Lu 1
Affiliation
|
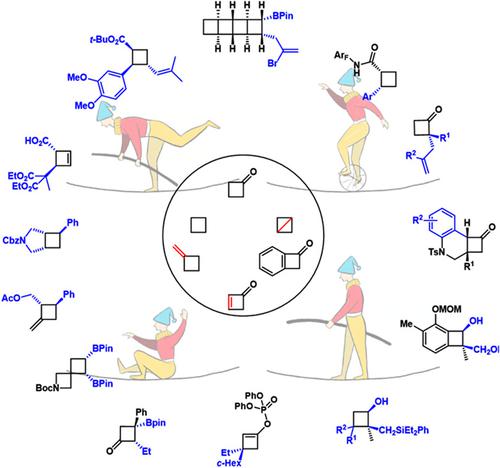
Cyclobutane derivatives have been recognized as useful structural motifs in organic synthesis and drug design. With the revival of photochemistry, the enantioselective synthesis of cyclobutane derivatives using [2 + 2]-cycloadditions has garnered numerous attentions. On the other hand, enantioselective functionalization of preformed four-membered carbocycles is emerging as an important complementary approach to access chiral cyclobutane derivatives with versatile structural patterns. Herein, we summarize recent advances in this field from 2012. To avoid undesired C—C bond cleavage driven by strain-releasing, it is crucial to choose compatible methods for enantioselective functionalization and meanwhile preserving intact four-membered ring skeleton. Guided by calculated hydrogenation enthalpies, which are used to evaluate the strain energy of indicated C—C bond, a clear picture of the developed methodologies on functionalization of four-membered carbocycles combining the strain energy and enhanced reactivity is presented.
中文翻译:

在绳索上跳舞 - 预制四元碳环的对映选择性功能化
环丁烷衍生物已被认为是有机合成和药物设计中有用的结构基序。随着光化学的复兴,利用[2+2]-环加成对映选择性合成环丁烷衍生物引起了广泛关注。另一方面,预形成的四元碳环的对映选择性功能化正在成为获得具有多种结构模式的手性环丁烷衍生物的重要补充方法。在此,我们总结了自 2012 年以来该领域的最新进展。为避免由应变释放驱动的不希望的 C-C 键断裂,选择兼容的方法进行对映选择性功能化并同时保持完整的四元环骨架至关重要。以计算的氢化焓为指导,
更新日期:2022-02-12

中文翻译:

在绳索上跳舞 - 预制四元碳环的对映选择性功能化
环丁烷衍生物已被认为是有机合成和药物设计中有用的结构基序。随着光化学的复兴,利用[2+2]-环加成对映选择性合成环丁烷衍生物引起了广泛关注。另一方面,预形成的四元碳环的对映选择性功能化正在成为获得具有多种结构模式的手性环丁烷衍生物的重要补充方法。在此,我们总结了自 2012 年以来该领域的最新进展。为避免由应变释放驱动的不希望的 C-C 键断裂,选择兼容的方法进行对映选择性功能化并同时保持完整的四元环骨架至关重要。以计算的氢化焓为指导,


































 京公网安备 11010802027423号
京公网安备 11010802027423号