Synlett ( IF 1.7 ) Pub Date : 2022-01-16 , DOI: 10.1055/a-1741-9069 Jaideep Saha 1 , Chenna Jagadeesh 1 , Biplab Mondal 1
|
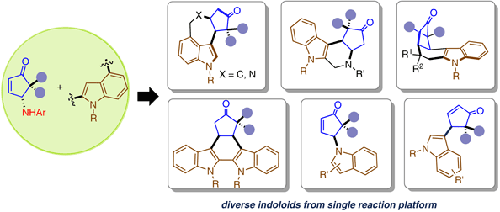
Capitalizing on the propensity of 1,2-amino group migration in γ-aminocyclopentenone with a suitable promoter, gem-diaryl-equipped systems unfolded an unprecedented avenue for the Lewis acid promoted displacement of γ-aniline group with nucleophiles such as indole. Such transformation, besides providing a means for direct γ-functionalization of cyclopentenones, presented innumerable scope for β,γ-annulation. Various tailored indolo bisnucleophiles were explored in the current study that rendered an array of indole alkaloid-like compounds in excellent yields and selectivity through one-pot operation. Analysis of collective experimental observation along with designed control experiments strongly suggested the possibility of a retro aza-Piancatelli rearrangement, which is hitherto unknown in the context. Such repertoire could find potential applications in the synthesis of complex assemblies from the Piancatelli rearrangement and related processes.
1 Introduction
2 Aza-Piancatelli Rearrangement and Related Domino Processes
3 An Unprecedented Aza-Piancatelli-Templated Strategy for Polycyclic Assemblies
4 Summary and Outlook
中文翻译:

从 4-氨基环戊烯酮开发 Aza-Piancatelli 模板化反应歧管:获得复杂的碳环组件
利用合适的促进剂gem来利用 γ-氨基环戊烯酮中 1,2-氨基迁移的倾向配备-二芳基的系统为路易斯酸促进用吲哚等亲核试剂置换γ-苯胺基团开辟了一条前所未有的途径。这种转变,除了为环戊烯酮的直接γ-官能化提供了一种手段外,还为β,γ-环化提供了无数的范围。在当前的研究中探索了各种定制的吲哚双亲核试剂,这些化合物通过一锅操作以优异的产率和选择性呈现了一系列吲哚生物碱类化合物。对集体实验观察的分析以及设计的对照实验强烈表明存在复古 aza-Piancatelli 重排的可能性,这在上下文中是迄今为止未知的。这样的曲目可以在从 Piancatelli 重排和相关过程合成复杂组件中找到潜在的应用。
1 简介
2 Aza-Piancatelli 重排和相关的多米诺骨牌过程
3 一种前所未有的多环组件的 Aza-Piancatelli 模板化策略
4 总结与展望































 京公网安备 11010802027423号
京公网安备 11010802027423号