Chemosphere ( IF 8.1 ) Pub Date : 2022-02-10 , DOI: 10.1016/j.chemosphere.2022.133907 Zhengnan Tu 1 , Yumeng Qi 1 , Ruijuan Qu 1 , Xiaosheng Tang 2 , Zunyao Wang 1 , Zongli Huo 3
|
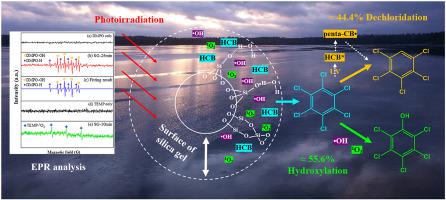
As one of the first batch of persistent organic pollutants (POPs) included in Stockholm Convention, hexachlorobenzene (HCB) has attracted great attention because of its wide occurrence and great environmental risks. Considering the easy adsorption of HCB on solids and the complexity of natural particles, we systematically investigated the photodegradation of HCB on the surface of silica gel (SG) in aqueous solution in this work to reveal its fate in natural waters. Under mercury lamp irradiation, more than 90% of HCB loaded on SG could be removed after 240 min. Moreover, the effects of solution pH and water constituents were examined, and results showed that the presence of NO2−, NO3−, Fe3+ and humic acid (HA) significantly inhibited the reaction due to the scavenging of ROS and/or competitive absorption of light. According to radical quenching experiments and electron paramagnetic resonance (EPR) spectra, hydroxyl radicals and singlet oxygen generated on the surface of SG could participate in the transformation of HCB, but •OH played a dominant role. Based on products identified by high performance liquid chromatography-mass spectrometry (HPLC-MS) and gas chromatography-mass spectrometry (GC-MS), two main pathways were proposed for the removal of HCB, including dechlorination and hydroxylation which represent direct and indirect photodegradation, respectively, and the occurrence of these two reactions was further supported by density functional theory (DFT) calculations. From the quantitative analysis of penta-chlorobenzene, it was estimated that dechlorination and hydroxylation contributed to approximately 44.4% and 55.6% of initial HCB degradation, respectively. Furthermore, toxicity predictions by the ecological structure–activity relationship model (ECOSAR) suggested that the toxicity of HCB was decreased in the photodegradation process. This study would provide important information for understanding the photochemical transformation mechanism of HCB at the solid/water interface.
中文翻译:

六氯苯 (HCB) 在固水系统中的光化学转化:动力学、机理和毒性评价
作为斯德哥尔摩公约首批列入的持久性有机污染物(POPs)之一,六氯苯(HCB)因其发生范围广、环境风险大而备受关注。考虑到六氯苯在固体上的易吸附性和天然颗粒的复杂性,我们系统地研究了六氯苯在水溶液中硅胶(SG)表面的光降解作用,以揭示其在天然水中的去向。在汞灯照射下,负载在 SG 上的 90% 以上的 HCB 可在 240 分钟后被去除。此外,研究了溶液pH值和水成分的影响,结果表明NO 2 -、NO 3 -、Fe 3+的存在由于 ROS 的清除和/或光的竞争性吸收,腐植酸 (HA) 显着抑制了反应。根据自由基猝灭实验和电子顺磁共振(EPR)光谱,SG表面产生的羟基自由基和单线态氧可以参与HCB的转化,但•OH起主要作用。基于高效液相色谱-质谱(HPLC-MS)和气相色谱-质谱(GC-MS)鉴定的产物,提出了去除HCB的两种主要途径,包括代表直接和间接光降解的脱氯和羟基化,分别,这两个反应的发生得到了密度泛函理论(DFT)计算的进一步支持。从五氯苯的定量分析,据估计,脱氯和羟基化分别促成了大约 44.4% 和 55.6% 的初始 HCB 降解。此外,生态构效关系模型(ECOSAR)的毒性预测表明,六氯苯的毒性在光降解过程中有所降低。本研究将为了解六氯苯在固/水界面的光化学转化机理提供重要信息。






























 京公网安备 11010802027423号
京公网安备 11010802027423号