Acta Pharmaceutica Sinica B ( IF 14.7 ) Pub Date : 2022-02-11 , DOI: 10.1016/j.apsb.2022.02.004 Youbo Zhang 1, 2 , Tingting Yan 2 , Tianxia Wang 1, 3 , Xiaoyan Liu 1 , Keisuke Hamada 2 , Dongxue Sun 2 , Yizheng Sun 1 , Yanfang Yang 1 , Jing Wang 1 , Shogo Takahashi 2 , Qiong Wang 2 , Kristopher W Krausz 2 , Changtao Jiang 4 , Cen Xie 2 , Xiuwei Yang 1 , Frank J Gonzalez 2
|
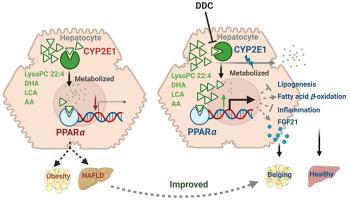
Although the functions of metabolic enzymes and nuclear receptors in controlling physiological homeostasis have been established, their crosstalk in modulating metabolic disease has not been explored. Genetic ablation of the xenobiotic-metabolizing cytochrome P450 enzyme CYP2E1 in mice markedly induced adipose browning and increased energy expenditure to improve obesity. CYP2E1 deficiency activated the expression of hepatic peroxisome proliferator-activated receptor alpha (PPARα) target genes, including fibroblast growth factor (FGF) 21, that upon release from the liver, enhanced adipose browning and energy expenditure to decrease obesity. Nineteen metabolites were increased in Cyp2e1-null mice as revealed by global untargeted metabolomics, among which four compounds, lysophosphatidylcholine and three polyunsaturated fatty acids were found to be directly metabolized by CYP2E1 and to serve as PPARα agonists, thus explaining how CYP2E1 deficiency causes hepatic PPARα activation through increasing cellular levels of endogenous PPARα agonists. Translationally, a CYP2E1 inhibitor was found to activate the PPARα–FGF21–beige adipose axis and decrease obesity in wild-type mice, but not in liver-specific Ppara-null mice. The present results establish a metabolic crosstalk between PPARα and CYP2E1 that supports the potential for a novel anti-obesity strategy of activating adipose tissue browning by targeting the CYP2E1 to modulate endogenous metabolites beyond its canonical role in xenobiotic-metabolism.
中文翻译:

CYP2E1 和 PPARα 底物和激动剂之间的串扰调节脂肪褐变和肥胖
尽管代谢酶和核受体在控制生理稳态中的功能已经确立,但它们在调节代谢疾病中的串扰尚未被探索。小鼠体内外源性代谢细胞色素 P450 酶 CYP2E1 的基因消融显着诱导脂肪褐变并增加能量消耗以改善肥胖。CYP2E1 缺乏会激活肝脏过氧化物酶体增殖物激活受体α (PPARα )靶基因的表达,包括成纤维细胞生长因子 (FGF) 21,这些基因在从肝脏释放后,会增强脂肪褐变和能量消耗,从而减少肥胖。Cyp2e1中增加了 19 种代谢物全球非靶向代谢组学揭示的-null小鼠,其中四种化合物,溶血磷脂酰胆碱和三种多不饱和脂肪酸被发现直接被CYP2E1代谢并充当PPARα激动剂,从而解释了CYP2E1缺乏如何通过增加细胞引起肝脏PPARα活化内源性 PPAR α激动剂的水平。在转化方面,发现 CYP2E1 抑制剂可激活 PPAR α -FGF21-米色脂肪轴并减少野生型小鼠的肥胖,但在肝脏特异性Ppara缺失小鼠中则不然。目前的结果建立了 PPAR α之间的代谢串扰和 CYP2E1,通过靶向 CYP2E1 来调节内源性代谢物,超出其在外源性代谢中的典型作用,从而支持激活脂肪组织褐变的新型抗肥胖策略的潜力。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号