当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Powerful Chiral Super Brønsted C–H Acid for Asymmetric Catalysis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-02-10 , DOI: 10.1021/jacs.1c12723 Bingfei Peng 1 , Jiguo Ma 1, 2 , Jianhua Guo 1 , Yating Gong 1 , Ronghao Wang 1 , Yi Zhang 1, 2 , Jinlong Zeng 1 , Wen-Wen Chen 1 , Kuiling Ding 2 , Baoguo Zhao 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2022-02-10 , DOI: 10.1021/jacs.1c12723 Bingfei Peng 1 , Jiguo Ma 1, 2 , Jianhua Guo 1 , Yating Gong 1 , Ronghao Wang 1 , Yi Zhang 1, 2 , Jinlong Zeng 1 , Wen-Wen Chen 1 , Kuiling Ding 2 , Baoguo Zhao 1
Affiliation
|
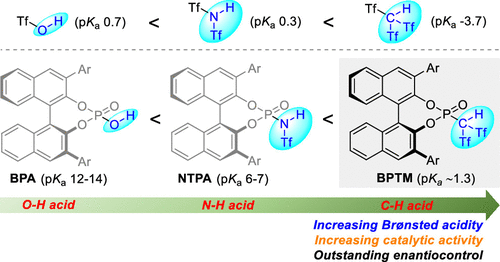
A new type of chiral super Brønsted C–H acids, BINOL-derived phosphoryl bis((trifluoromethyl)sulfonyl) methanes (BPTMs), were developed. As compared to widely utilized BINOL-derived chiral phosphoric acids (BPAs) and N-triflyl phosphoramides (NTPAs), BPTMs displayed much higher Brønsted acidity, resulting in dramatically improved activity and excellent enantioselectivity as demonstrated in catalytic asymmetric Mukaiyama–Mannich reaction, allylic amination, three-component coupling of allyltrimethylsilane with 9-fluorenylmethyl carbamate and aldehydes, and protonation of silyl enol ether. These new strong Brønsted C–H acids have provided a platform for expanding the chemistry of asymmetric Brønsted acid catalysis.
中文翻译:

用于不对称催化的强大手性超级布朗斯台德 C-H 酸
开发了一种新型手性超级布朗斯台德 C-H 酸,即 BINOL 衍生的磷酸双((三氟甲基)磺酰基)甲烷 (BPTMs)。与广泛使用的 BINOL 衍生的手性磷酸 (BPA) 和N -triflyl 磷酰胺 (NTPAs) 相比,BPTM 表现出更高的布朗斯台德酸度,从而显着提高活性和优异的对映选择性,如催化不对称 Mukaiyama-Mannich 反应、烯丙基胺化,烯丙基三甲基硅烷与 9-芴基甲基氨基甲酸酯和醛的三组分偶联,以及甲硅烷基烯醇醚的质子化。这些新的强 Brønsted C-H 酸为扩展不对称 Brønsted 酸催化的化学性质提供了一个平台。
更新日期:2022-02-10
中文翻译:

用于不对称催化的强大手性超级布朗斯台德 C-H 酸
开发了一种新型手性超级布朗斯台德 C-H 酸,即 BINOL 衍生的磷酸双((三氟甲基)磺酰基)甲烷 (BPTMs)。与广泛使用的 BINOL 衍生的手性磷酸 (BPA) 和N -triflyl 磷酰胺 (NTPAs) 相比,BPTM 表现出更高的布朗斯台德酸度,从而显着提高活性和优异的对映选择性,如催化不对称 Mukaiyama-Mannich 反应、烯丙基胺化,烯丙基三甲基硅烷与 9-芴基甲基氨基甲酸酯和醛的三组分偶联,以及甲硅烷基烯醇醚的质子化。这些新的强 Brønsted C-H 酸为扩展不对称 Brønsted 酸催化的化学性质提供了一个平台。













































 京公网安备 11010802027423号
京公网安备 11010802027423号