Synthesis ( IF 2.2 ) Pub Date : 2022-02-09 , DOI: 10.1055/s-0040-1719886 József Kupai 1 , Gyula Dargó 1 , Sándor Nagy 1 , Dávid Kis 1 , Péter Bagi 1 , Béla Mátravölgyi 1 , Blanka Tóth 2 , Péter Huszthy 1 , László Drahos 3
|
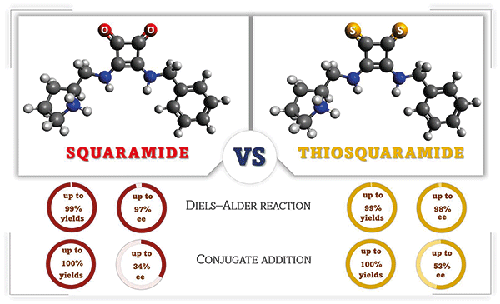
The synthesis of chiral proline-derived squaramide and thiosquaramide organocatalysts, which are capable of the dual activation in asymmetric reactions is reported. The (thio)squaramide moiety can form hydrogen bonds to activate the substrates and to stereocontrol the reaction, while the pyrrolidine unit can form enamines to activate carbonyl compounds via aminocatalysis. Comparing the performance of thiosquaramide to squaramide, the Diels–Alder reaction of (anthracen-9-yl)acetaldehyde and trans-β-nitrostyrene was examined, which has been investigated in the literature using quantum chemical calculations. Both squaramide and thiosquaramide gave excellent yields (up to 99%) and enantiomeric excess values (up to 98%). Moreover, their catalytic performance was compared in conjugate addition of lawsone to β,γ-unsaturated α-keto ester.
中文翻译:

脯氨酸衍生(硫代)方酰胺有机催化剂在不对称 Diels-Alder 和共轭加成反应中的应用
报道了手性脯氨酸衍生的方方酰胺和硫方方酰胺有机催化剂的合成,它们能够在不对称反应中进行双重活化。(硫代)方酰胺部分可以形成氢键以活化底物并立体控制反应,而吡咯烷单元可以形成烯胺以通过氨基催化活化羰基化合物。硫代角鲨酰胺与角鲨酰胺的性能比较,(anthracen-9-yl) 乙醛和反式的 Diels-Alder 反应对-β-硝基苯乙烯进行了检查,这已在文献中使用量子化学计算进行了研究。方方酰胺和硫方方酰胺都具有优异的产率(高达 99%)和对映体过量值(高达 98%)。此外,比较了它们在将指甲花酮与β,γ-不饱和α-酮酯共轭加成时的催化性能。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号