当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Green Closed-Loop Cathode Regeneration from Spent NMC-Based Lithium-Ion Batteries through Bioleaching
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-02-09 , DOI: 10.1021/acssuschemeng.1c06885 Minh Phuong Do 1, 2 , Joseph Jegan Roy 1, 2, 3 , Bin Cao 3, 4 , Madhavi Srinivasan 1, 2
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2022-02-09 , DOI: 10.1021/acssuschemeng.1c06885 Minh Phuong Do 1, 2 , Joseph Jegan Roy 1, 2, 3 , Bin Cao 3, 4 , Madhavi Srinivasan 1, 2
Affiliation
|
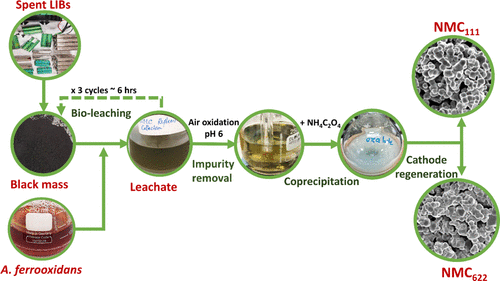
Addressing the growing volume of end-of-life lithium-ion battery (LIB) waste is one of the global challenges in tackling the electronic waste problem. In this study, the regeneration of LiNi0.3Co0.3Mn0.3O2 (NMC111) and LiNi0.6Co0.2Mn0.2O2 (NMC622) cathode-active materials from end-of-life LIBs was accomplished through an environmentally friendly bioleaching process. In the bioleaching process mediated by Acidithiobacillus ferrooxidans, 85.5% of Ni, 91.8% of Mn, 90.4% of Co, and 89.9% of Li were leached out from NMC-based spent LIBs in 6 h at a pulp density of 100 g/L. One of the challenges in bioleaching-based metal recovery is the presence of impurities, including Cu, Al, and Fe (excess Fe3+ and Fe2+ from bacterial nutrients). The impurity removal was performed by air oxidation and pH adjustment without substantial losses of other metallic ions. Thereafter, ammonium oxalate coprecipitation effectively recovered the transition metal ions as metal oxalates from the bioleaching liquor. NMC111 and NMC622 were regenerated from the coprecipitated product. The electrochemical stability of the regenerated NMC111 and NMC622 was comparable to commercial NMC (∼85% of capacity retention after 50 cycles at 100 mA g–1). This regeneration approach appears promising in LIB recycling for long-term industrial development.
中文翻译:

通过生物浸出废旧 NMC 锂离子电池的绿色闭环阴极再生
解决越来越多的报废锂离子电池 (LIB) 废物是解决电子废物问题的全球挑战之一。在这项研究中,LiNi 0.3 Co 0.3 Mn 0.3 O 2 (NMC 111 ) 和 LiNi 0.6 Co 0.2 Mn 0.2 O 2 (NMC 622 ) 正极活性材料从报废的 LIBs 中通过环保生物浸出再生。过程。在氧化亚铁硫杆菌介导的生物浸出过程中85.5% 的镍、91.8% 的锰、90.4% 的钴和 89.9% 的锂在 6 小时内从 NMC 基废锂离子电池中浸出,纸浆密度为 100 g/L。基于生物浸出的金属回收的挑战之一是杂质的存在,包括 Cu、Al 和 Fe(来自细菌营养物的过量 Fe 3+和 Fe 2+ )。通过空气氧化和pH调节来去除杂质,而不会大量损失其他金属离子。此后,草酸铵共沉淀有效地将过渡金属离子作为金属草酸盐从生物浸出液中回收。NMC 111和 NMC 622由共沉淀产物再生。再生 NMC 111和 NMC 622的电化学稳定性与商业 NMC 相当(在 100 mA g -1下 50 次循环后容量保持率约为 85% )。这种再生方法在长期工业发展的 LIB 回收中似乎很有前景。
更新日期:2022-02-09
中文翻译:

通过生物浸出废旧 NMC 锂离子电池的绿色闭环阴极再生
解决越来越多的报废锂离子电池 (LIB) 废物是解决电子废物问题的全球挑战之一。在这项研究中,LiNi 0.3 Co 0.3 Mn 0.3 O 2 (NMC 111 ) 和 LiNi 0.6 Co 0.2 Mn 0.2 O 2 (NMC 622 ) 正极活性材料从报废的 LIBs 中通过环保生物浸出再生。过程。在氧化亚铁硫杆菌介导的生物浸出过程中85.5% 的镍、91.8% 的锰、90.4% 的钴和 89.9% 的锂在 6 小时内从 NMC 基废锂离子电池中浸出,纸浆密度为 100 g/L。基于生物浸出的金属回收的挑战之一是杂质的存在,包括 Cu、Al 和 Fe(来自细菌营养物的过量 Fe 3+和 Fe 2+ )。通过空气氧化和pH调节来去除杂质,而不会大量损失其他金属离子。此后,草酸铵共沉淀有效地将过渡金属离子作为金属草酸盐从生物浸出液中回收。NMC 111和 NMC 622由共沉淀产物再生。再生 NMC 111和 NMC 622的电化学稳定性与商业 NMC 相当(在 100 mA g -1下 50 次循环后容量保持率约为 85% )。这种再生方法在长期工业发展的 LIB 回收中似乎很有前景。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号