当前位置:
X-MOL 学术
›
J. Mol. Liq.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorption performance of C12, B6N6 and Al6N6 nanoclusters towards hazardous gas molecules: A DFT investigation for gas sensing and removal application
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2022-02-10 , DOI: 10.1016/j.molliq.2022.118702 Saurav Patel 1 , Paras Patel 1 , Darshil Chodvadiya 1 , Narayan N. Som 2 , Prafulla K. Jha 1
Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2022-02-10 , DOI: 10.1016/j.molliq.2022.118702 Saurav Patel 1 , Paras Patel 1 , Darshil Chodvadiya 1 , Narayan N. Som 2 , Prafulla K. Jha 1
Affiliation
|
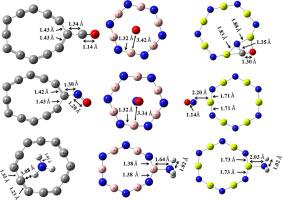
Nowadays, it is very important to make suitable materials that are capable of detection of toxic gas molecules (CO, NO and NH3 ) as well as their disposal from the atmosphere to reduce environmental damage. In the present work, we have analyzed structural, electronic and sensing properties to understand the adsorption mechanism of pristine C12 , B6 N6 and Al6 N6 nanoclusters towards toxic gas molecules using density functional theory. The C12 nanocluster towards CO and NO gas molecules, B6 N6 nanocluster towards NH3 gas molecule and Al6 N6 nanocluster towards CO, NO and NH3 gas molecules show chemisorption nature, whereas C12 nanocluster towards NH3 gas molecule and B6 N6 nanocluster towards CO and NO gas molecules show physisorption nature. The interaction between gas molecules and nanoclusters results into the charge redistribution which induces the dipole moment. The electronic conductivity and work function are modified due to the change in the position of the HOMO and LUMO energies. Our results show that the C12 nanocluster for CO and NO gas molecules, B6 N6 nanocluster for NH3 gas molecule, Al6 N6 nanocluster for NO gas molecule can be used as an electronic sensor while C12 nanocluster for NO gas molecule, B6 N6 nanocluster for NH3 gas molecule, Al6 N6 nanocluster for NH3 gas molecule can served as a φ 12 nanocluster for CO and NO gas molecules, B6 N6 nanocluster for NH3 gas molecule and Al6 N6 nanocluster for CO and NH3 gas molecules can be used for gas removal from the environment. However, due to optimal interaction and shorter recovery time, Al6 N6 nanocluster can be used as NO gas sensor. Our results clearly indicate the use of these three nanoclusters for designing and developing a promising gas sensor device.
中文翻译:

C12、B6N6 和 Al6N6 纳米团簇对有害气体分子的吸附性能:用于气体传感和去除应用的 DFT 研究
如今,制造能够检测有毒气体分子(CO、NO 和 NH3)以及从大气中处理它们以减少环境破坏的合适材料非常重要。在本工作中,我们分析了结构、电子和传感特性,以利用密度泛函理论了解原始 C12、B6N6 和 Al6N6 纳米团簇对有毒气体分子的吸附机制。朝向 CO 和 NO 气体分子的 C12 纳米团簇、朝向 NH3 气体分子的 B6N6 纳米团簇和朝向 CO、NO 和 NH3 气体分子的 Al6N6 纳米团簇表现出化学吸附性质,而朝向 NH3 气体分子的 C12 纳米团簇和朝向 CO 和 NO 气体分子的 B6N6 纳米团簇表现出物理吸附性质。气体分子和纳米团簇之间的相互作用导致电荷重新分布,从而感应出偶极矩。由于 HOMO 和 LUMO 能量位置的变化,电子电导率和功函数被改变。我们的结果表明,用于 CO 和 NO 气体分子的 C12 纳米团簇、用于 NH3 气体分子的 B6N6 纳米团簇、用于 NO 气体分子的 Al6N6 纳米团簇可用作电子传感器,而用于 NO 气体分子的 C12 纳米团簇、用于 NH3 气体分子的 B6N6 纳米团簇、用于 NH3 气体分子的 Al6N6 纳米团簇可用作φ型传感器。由于相互作用非常强且恢复时间较长,用于 CO 和 NO 气体分子的 C12 纳米团簇、用于 NH3 气体分子的 B6N6 纳米团簇和用于 CO 和 NH3 气体分子的 Al6N6 纳米团簇可用于从环境中去除气体。然而,由于最佳相互作用和更短的恢复时间,Al6N6 纳米团簇可用作 NO 气体传感器。 我们的结果清楚地表明,这三个纳米团簇被用于设计和开发一种有前途的气体传感器设备。
更新日期:2022-02-10
中文翻译:

C12、B6N6 和 Al6N6 纳米团簇对有害气体分子的吸附性能:用于气体传感和去除应用的 DFT 研究
如今,制造能够检测有毒气体分子(CO、NO 和 NH3)以及从大气中处理它们以减少环境破坏的合适材料非常重要。在本工作中,我们分析了结构、电子和传感特性,以利用密度泛函理论了解原始 C12、B6N6 和 Al6N6 纳米团簇对有毒气体分子的吸附机制。朝向 CO 和 NO 气体分子的 C12 纳米团簇、朝向 NH3 气体分子的 B6N6 纳米团簇和朝向 CO、NO 和 NH3 气体分子的 Al6N6 纳米团簇表现出化学吸附性质,而朝向 NH3 气体分子的 C12 纳米团簇和朝向 CO 和 NO 气体分子的 B6N6 纳米团簇表现出物理吸附性质。气体分子和纳米团簇之间的相互作用导致电荷重新分布,从而感应出偶极矩。由于 HOMO 和 LUMO 能量位置的变化,电子电导率和功函数被改变。我们的结果表明,用于 CO 和 NO 气体分子的 C12 纳米团簇、用于 NH3 气体分子的 B6N6 纳米团簇、用于 NO 气体分子的 Al6N6 纳米团簇可用作电子传感器,而用于 NO 气体分子的 C12 纳米团簇、用于 NH3 气体分子的 B6N6 纳米团簇、用于 NH3 气体分子的 Al6N6 纳米团簇可用作φ型传感器。由于相互作用非常强且恢复时间较长,用于 CO 和 NO 气体分子的 C12 纳米团簇、用于 NH3 气体分子的 B6N6 纳米团簇和用于 CO 和 NH3 气体分子的 Al6N6 纳米团簇可用于从环境中去除气体。然而,由于最佳相互作用和更短的恢复时间,Al6N6 纳米团簇可用作 NO 气体传感器。 我们的结果清楚地表明,这三个纳米团簇被用于设计和开发一种有前途的气体传感器设备。































 京公网安备 11010802027423号
京公网安备 11010802027423号