Colloids and Surfaces A: Physicochemical and Engineering Aspects ( IF 4.9 ) Pub Date : 2022-02-10 , DOI: 10.1016/j.colsurfa.2022.128561 Wenming Wu 1 , Jirui Hou 1 , Gang Li 2 , Lifeng Chen 2
|
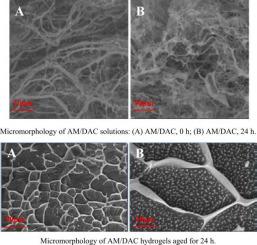
Compared with ordinary polyacrylamide, acrylamide copolymer of acryloyloxyethyl trimethylammonium chloride (AM/DAC) hydrogel is easier to reside in large pores and fractures in sandstone formation through electrostatic adsorption. However, the syneresis mechanism of AM/DAC hydrogel caused by high temperature and inorganic salts has not been clear. In this paper, the type of water in gelling solutions and hydrogels was analyzed by the DSC method, the results showed that the bound water in the hydrogel was transformed into free water and then the free water was separated from the hydrogel during the syneresis of AM/DAC hydrogel. Experimental investigations, including dynamic light scattering (DLS), differential scanning calorimetry (DSC), high-performance liquid chromatography (HPLC), phase-analysis light-scattering (PALS), and scanning electron microscope (SEM), have been conducted to elucidate the mechanism of temperature and inorganic salts on syneresis rate of AM/DAC hydrogel. The result showed that with the increase of temperature, the polymer in the AM/DAC hydrogel degraded and the molecular chain broken, which destroyed the grid of hydrogel and reduced the water holding capacity. At the same time, the formation of the hydrophobic group in the molecular chain led to the decrease of the hydrophilicity so that the AM/DAC hydrogel was easy to syneresis. In addition, the increase of temperature will promote the formation of crosslinking intermediate (di/trimethylol), improve the crosslinking rate between crosslinking intermediate and polymer, which makes the AM/DAC hydrogel easy to over crosslink. Inorganic salts diffused the electric double layer by compressing the polymer and competed with the hydrophilic groups on the polymer to absorb water, which will reduce the elongation and hydrophilicity of the polymer molecular chain. This will lead to the decrease of the hydrodynamic radius of polymer molecules in AM/DAC hydrogel, which is the main reason why inorganic salts promote hydrogel dehydration. Besides, inorganic salts had little effect on the formation rate and crosslinking rate of di/trimethylol. Therefore, reducing the molecular hydrodynamic radius of AM/DAC is the main reason why inorganic salts promote the syneresis of AM/DAC hydrogel. The research demonstrated the mechanism of high temperature and inorganic salts on syneresis rate of AM/DAC hydrogel. This study also provides technical guidelines for inhibiting hydrogel syneresis.
中文翻译:

温度和无机盐浓度对AM/DAC水凝胶脱水收缩率的影响
与普通聚丙烯酰胺相比,丙烯酰氧基乙基三甲基氯化铵(AM/DAC)水凝胶的丙烯酰胺共聚物通过静电吸附更容易滞留在砂岩地层的大孔隙和裂缝中。然而,高温和无机盐引起的AM/DAC水凝胶的脱水收缩机制尚不清楚。本文采用 DSC 方法对凝胶溶液和水凝胶中水的类型进行了分析,结果表明在 AM 的脱水收缩过程中,水凝胶中的结合水转化为游离水,然后游离水从水凝胶中分离出来。 /DAC 水凝胶。实验研究,包括动态光散射 (DLS)、差示扫描量热法 (DSC)、高效液相色谱 (HPLC)、相分析光散射 (PALS)、和扫描电子显微镜 (SEM), 以阐明温度和无机盐对 AM/DAC 水凝胶脱水率的机制。结果表明,随着温度的升高,AM/DAC水凝胶中的聚合物降解,分子链断裂,破坏了水凝胶的网格,降低了水凝胶的持水能力。同时,分子链中疏水基团的形成导致亲水性降低,使AM/DAC水凝胶易于脱水收缩。此外,温度的升高会促进交联中间体(二/三羟甲基)的形成,提高交联中间体与聚合物的交联率,使AM/DAC水凝胶容易过度交联。无机盐通过压缩聚合物扩散双电层,与聚合物上的亲水基团竞争吸水,降低聚合物分子链的伸长率和亲水性。这将导致AM/DAC水凝胶中聚合物分子的流体动力学半径减小,这是无机盐促进水凝胶脱水的主要原因。此外,无机盐对二/三羟甲基的生成速率和交联速率影响不大。因此,减小AM/DAC的分子流体动力学半径是无机盐促进AM/DAC水凝胶脱水收缩的主要原因。研究证实了高温和无机盐对AM/DAC水凝胶脱水率的影响机制。该研究还提供了抑制水凝胶脱水收缩的技术指南。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号