当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Effect of Methylamine, Amylamine, and Decylamine on the Formation and Dissociation Kinetics of CO2 Hydrate Relevant for Carbon Dioxide Sequestration
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-02-09 , DOI: 10.1021/acs.iecr.1c04074 Chandan Sahu 1, 2, 3, 4 , Anirbid Sircar 4 , Jitendra S. Sangwai 1, 2, 3 , Rajnish Kumar 2, 3
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2022-02-09 , DOI: 10.1021/acs.iecr.1c04074 Chandan Sahu 1, 2, 3, 4 , Anirbid Sircar 4 , Jitendra S. Sangwai 1, 2, 3 , Rajnish Kumar 2, 3
Affiliation
|
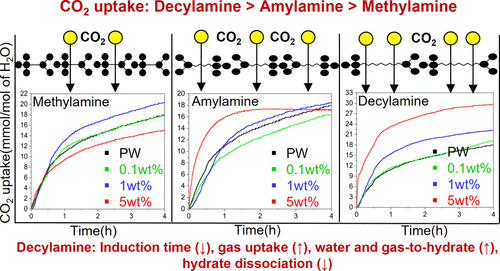
Gas hydrates have been the nucleus of research from a sustainable engineering standpoint, considering their unique applications in a broad spectrum of scientific contexts. One such application is the sequestration of gaseous CO2 as solid hydrates under the seabed. Low temperature and high pressure are prevalent below the seabed, making it a thermodynamically feasible process. Furthermore, improved CO2 hydrate kinetics will facilitate technological development for carbon capture, storage, and sequestration. This study focuses on comprehending the CO2 hydrate kinetics with organic aliphatic amines, particularly methylamine, amylamine, and decylamine. Additives were tested in concentrations of 0.1, 1, and 5 wt % to meticulously comprehend their impact. A 300 mL stirred tank reactor was used for the investigations at 3.5 MPa and 274.55 K with pure water, which are the typical temperature and pressure conditions that one encounters in shallow subsea sediments. All additives showed considerable promotion in induction time, assuring faster CO2 hydrate nucleation. In addition, decylamine resulted in faster uptake of CO2 in our experiments compared to the other two additives. Hydrate dissociation studies up to 293.15 K were performed to assess the effect of the considered additives on CO2 hydrate dissociation. The decylamine system also delayed the gas release rate, showing better stability than the pure water system. This study also proposes a suitable well design for enhanced subsea CO2 sequestration as solid hydrates.
中文翻译:

甲胺、戊胺和癸胺对二氧化碳封存相关 CO2 水合物形成和解离动力学的影响
从可持续工程的角度来看,天然气水合物一直是研究的核心,考虑到它们在广泛的科学环境中的独特应用。一种这样的应用是将气态CO 2封存为海床下的固体水合物。海底以下普遍存在低温和高压,使其成为热力学可行的过程。此外,改进的 CO 2水合物动力学将促进碳捕获、储存和封存的技术发展。本研究的重点是理解 CO 2与有机脂肪胺,特别是甲胺、戊胺和癸胺的水合物动力学。添加剂以 0.1、1 和 5 wt% 的浓度进行了测试,以仔细了解它们的影响。使用 300 mL 搅拌罐式反应器在 3.5 MPa 和 274.55 K 下用纯水进行研究,这是人们在浅海沉积物中遇到的典型温度和压力条件。所有添加剂在诱导时间方面都表现出相当大的提升,确保更快的 CO 2水合物成核。此外,与其他两种添加剂相比,在我们的实验中,癸胺导致 CO 2的吸收更快。进行了高达 293.15 K 的水合物解离研究,以评估所考虑的添加剂对 CO 2的影响水合物离解。癸胺体系也延缓了气体释放速率,表现出比纯水体系更好的稳定性。该研究还提出了一种合适的井设计,以增强海底 CO 2固体水合物的封存。
更新日期:2022-02-09
中文翻译:

甲胺、戊胺和癸胺对二氧化碳封存相关 CO2 水合物形成和解离动力学的影响
从可持续工程的角度来看,天然气水合物一直是研究的核心,考虑到它们在广泛的科学环境中的独特应用。一种这样的应用是将气态CO 2封存为海床下的固体水合物。海底以下普遍存在低温和高压,使其成为热力学可行的过程。此外,改进的 CO 2水合物动力学将促进碳捕获、储存和封存的技术发展。本研究的重点是理解 CO 2与有机脂肪胺,特别是甲胺、戊胺和癸胺的水合物动力学。添加剂以 0.1、1 和 5 wt% 的浓度进行了测试,以仔细了解它们的影响。使用 300 mL 搅拌罐式反应器在 3.5 MPa 和 274.55 K 下用纯水进行研究,这是人们在浅海沉积物中遇到的典型温度和压力条件。所有添加剂在诱导时间方面都表现出相当大的提升,确保更快的 CO 2水合物成核。此外,与其他两种添加剂相比,在我们的实验中,癸胺导致 CO 2的吸收更快。进行了高达 293.15 K 的水合物解离研究,以评估所考虑的添加剂对 CO 2的影响水合物离解。癸胺体系也延缓了气体释放速率,表现出比纯水体系更好的稳定性。该研究还提出了一种合适的井设计,以增强海底 CO 2固体水合物的封存。








































 京公网安备 11010802027423号
京公网安备 11010802027423号