当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of 2,6-Dimethyltyrosine-Like Amino Acids through Pinacolinamide-Enabled C–H Dimethylation of 4-Dibenzylamino Phenylalanine
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-02-09 , DOI: 10.1021/acs.joc.1c02527 Davide Illuminati 1, 2 , Anna Fantinati 3 , Tiziano De Ventura 1 , Daniela Perrone 3 , Chiara Sturaro 4 , Valentina Albanese 1 , Erika Marzola 1 , Virginia Cristofori 1 , Julie Oble 2 , Giovanni Poli 2 , Claudio Trapella 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2022-02-09 , DOI: 10.1021/acs.joc.1c02527 Davide Illuminati 1, 2 , Anna Fantinati 3 , Tiziano De Ventura 1 , Daniela Perrone 3 , Chiara Sturaro 4 , Valentina Albanese 1 , Erika Marzola 1 , Virginia Cristofori 1 , Julie Oble 2 , Giovanni Poli 2 , Claudio Trapella 1
Affiliation
|
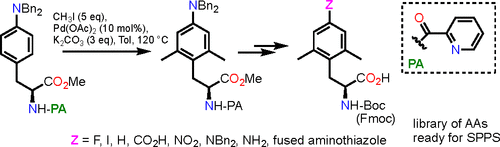
The synthesis of a small library of NH-Boc- or NH-Fmoc-protected l-phenylalanines carrying methyl groups at positions 2 and 6 and diverse functionalities at position 4 has been achieved. The approach, which took advantage of a Pd-catalyzed directed C–H dimethylation of picolinamide derivatives, allowed the electronic and steric properties of the resulting amino acid derivatives to be altered by appending a variety of electron-withdrawing, electron-donating, or bulky groups.
中文翻译:

通过 4-二苄基氨基苯丙氨酸的频哪醇酰胺 C-H 二甲基化合成 2,6-二甲基酪氨酸样氨基酸
已经实现了在 2 位和 6 位带有甲基基团和在 4 位具有多种官能团的NH -Boc-或NH -Fmoc-保护的 L-苯丙氨酸的小型文库的合成。该方法利用 Pd 催化的甲基吡啶酰胺衍生物的定向 C-H 二甲基化,通过添加各种吸电子、给电子或庞大的物质,可以改变所得氨基酸衍生物的电子和空间特性。团体。
更新日期:2022-02-09
中文翻译:

通过 4-二苄基氨基苯丙氨酸的频哪醇酰胺 C-H 二甲基化合成 2,6-二甲基酪氨酸样氨基酸
已经实现了在 2 位和 6 位带有甲基基团和在 4 位具有多种官能团的NH -Boc-或NH -Fmoc-保护的 L-苯丙氨酸的小型文库的合成。该方法利用 Pd 催化的甲基吡啶酰胺衍生物的定向 C-H 二甲基化,通过添加各种吸电子、给电子或庞大的物质,可以改变所得氨基酸衍生物的电子和空间特性。团体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号