Journal of Environmental Management ( IF 8.0 ) Pub Date : 2022-02-05 , DOI: 10.1016/j.jenvman.2022.114631 Guangyu Duan 1 , Zhanfang Cao 1 , Hong Zhong 1 , Xin Ma 1 , Shuai Wang 1
|
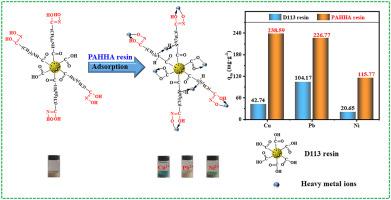
Heavy metal wastewater pollution has become an ecological challenge worldwide. This study reports the development of a novel poly (6-acryloylamino-N-hydroxyhexanamide) (PAHHA) resin for effective adsorption of heavy metal ions, including Cu2+, Pb2+ and Ni2+. The chelating resin was synthesized by the grafting reaction between 6-amino-N-hydroxyhexanamide and polyacrylic resin, thus containing the hydroxamate and acylamino groups. The batch adsorption experiments revealed that the PAHHA resin exhibited an excellent adsorption performance for Cu2+, Pb2+ and Ni2+. The maximum adsorption capacities of Cu2+, Pb2+ and Ni2+ were determined to be 238.59, 232.48 and 115.77 mg·g−1, respectively. Based on the adsorption kinetics, the pseudo-second-order kinetic model was noted to fit well for all metal ions. The metal ion concentration as a function of the equilibrium adsorption capacity fitted well with the Langmuir isotherm, thus indicating the single layer adsorption process. The adsorption mechanism was investigated by using Fourier transform infrared spectroscopy (FT-IR), scanning electron microscope (SEM), density functional theory (DFT) calculations, X-ray photoelectron spectroscopy (XPS) and adsorption isotherms. It was revealed that the PAHHA resin possessed multiple active sites, including –CONHOH, –CONH– and –COOH, which could strongly adsorb the metal ions. Specifically, the –CONHOH group displayed a high affinity by forming a stable five-membered ring with heavy metal ions. Overall, the developed resin exhibits advantages such as simple synthesis, inexpensive raw material and good recyclability, along with high adsorption ability, thus providing a new approach for efficiently treating wastewater contaminated with heavy metal ions.
中文翻译:

用于吸附重金属离子的高效聚(6-丙烯酰氨基-N-羟基己酰胺)树脂
重金属废水污染已成为世界范围内的生态挑战。本研究报告了一种新型聚(6-丙烯酰氨基-N-羟基己酰胺)(PAHHA)树脂的开发,该树脂可有效吸附重金属离子,包括Cu 2+、Pb 2+和Ni 2+。螯合树脂是通过6-氨基-N-羟基己酰胺与聚丙烯酸树脂的接枝反应合成的,因此含有异羟肟酸酯和酰氨基。批量吸附实验表明,PAHHA树脂对Cu 2+、Pb 2+和Ni 2+表现出优异的吸附性能。Cu 2+、Pb 2+和Ni 2+的最大吸附容量测定为 238.59、232.48 和 115.77 mg·g -1, 分别。基于吸附动力学,准二级动力学模型被认为非常适合所有金属离子。金属离子浓度作为平衡吸附容量的函数与朗缪尔等温线拟合良好,从而表明单层吸附过程。采用傅里叶变换红外光谱(FT-IR)、扫描电子显微镜(SEM)、密度泛函理论(DFT)计算、X射线光电子能谱(XPS)和吸附等温线研究吸附机理。结果表明,PAHHA树脂具有多个活性位点,包括-CONHOH、-CONH-和-COOH,可以强烈吸附金属离子。具体而言,-CONHOH 基团通过与重金属离子形成稳定的五元环显示出高亲和力。全面的,






























 京公网安备 11010802027423号
京公网安备 11010802027423号