Chemistry of Heterocyclic Compounds ( IF 1.4 ) Pub Date : 2022-02-04 , DOI: 10.1007/s10593-022-03051-4 Alexander V. Komkov 1 , Mikhail A. Kozlov 1 , Darina I. Nasyrova 1, 2 , Andrey S. Dmitrenok 1 , Igor V. Zavarzin 1
|
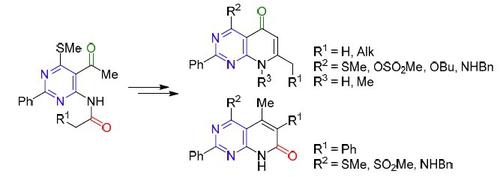
Methods were developed for the synthesis of new pyrido[2,3-d]pyrimidin-5-ones and pyrido[2,3-d]pyrimidin-7-ones from 5-acetyl-N-acyl-6-amino-4-methylsulfanyl-2-phenylpyrimidines. Heating the latter with MeONa at reflux in BuOH led to selective formation of pyrido[2,3-d]pyrimidin-5-ones or pyrido[2,3-d]pyrimidin-7-ones (containing an intact SMe group or resulting in its substitution with OBu group), depending on the nature of the acyl group. The possible substitution of SMe and OBu groups in pyridopyrimidinones was explored by using BnNH2. It was demonstrated that prior oxidation of SMe group with m-chloroperbenzoic acid allowed for simpler introduction of the NHBn moiety.
中文翻译:

从 5-乙酰基-6-氨基-4-甲基硫烷基-2-苯基嘧啶合成新的吡啶并[2,3-d]嘧啶-5-酮和吡啶并[2,3-d]嘧啶-7-酮在 4 位功能化
开发了从 5-乙酰基-N-酰基-6-氨基-4-合成新的吡啶并[2,3 - d ]嘧啶-5-酮和吡啶并[2,3 - d ]嘧啶-7-酮的方法甲硫基-2-苯基嘧啶。在 BuOH 中用 MeONa 加热后者,导致选择性形成 pyrido[2,3 - d ]pyrimidin-5-ones 或 pyrido[2,3- d ]pyrimidin-7-ones(含有完整的 SMe 基团或导致它被 OBu 基团取代),取决于酰基的性质。通过使用BnNH 2探索了吡啶并嘧啶酮中SMe和OBu基团的可能取代。已经证明,SMe 基团的预先氧化与m-氯过苯甲酸可以更简单地引入 NHBn 部分。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号