Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2022-02-04 , DOI: 10.1016/j.jfluchem.2022.109950 Tongyun Zhang 1 , Chengping Zhang 2 , Xiaoxun Ma 1 , Hengdao Quan 2
|
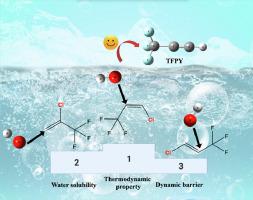
Chlorotrifluoropropene isomers including 1‑chloro-3,3,3-trifluoroproene (HCFO-1233zd(Z/E)) and 2‑chloro-3,3,3-trifluoropropene (HCFO-1233xf) were adopted as the dehydrochlorination materials to produce 3,3,3-trifluoropropyne (TFPY) by one-pot method were reported. The experimental data indicated that HCFO-1233zd(Z) was the most favorable synthetic raw material in this process, followed by HCFO-1233xf, and HCFO-1233zd(E) then. However, DFT calculations demonstrated the superiority of HCFO-1233zd(E) to HCFO-1233xf according to the reaction energy barrier. By further referring to the thermodynamic calculations and the computed water solubility, the reaction priority herein was identified as HCFO-1233zd(Z) > HCFO-1233xf > HCFO-1233zd(E). Therefore, compared to kinetic effects, the reactivity of HCFO-1233zd(Z/E) and HCFO-1233xf was evidently determined by their thermodynamic properties and solubility. This work thus provides guidance for similar hydrogen halide removal in liquid phase.
中文翻译:

三氟氯丙烯异构体液相合成3,3,3-三氟丙炔
采用氯三氟丙烯异构体包括 1-氯-3,3,3-三氟丙烯 (HCFO-1233zd(Z/E)) 和 2-氯-3,3,3-三氟丙烯 (HCFO-1233xf) 作为脱氯化氢原料生产 3 ,3,3-三氟丙炔 (TFPY) 通过一锅法进行了报道。实验数据表明HCFO-1233zd(Z)是该工艺最有利的合成原料,其次是HCFO-1233xf,其次是HCFO-1233zd(E)。然而,根据反应能垒,DFT 计算证明 HCFO-1233zd(E) 优于 HCFO-1233xf。通过进一步参考热力学计算和计算出的水溶性,本文的反应优先级确定为HCFO-1233zd(Z) > HCFO-1233xf > HCFO-1233zd(E)。因此,与动力学效应相比,HCFO-1233zd(Z/E) 和 HCFO-1233xf 的反应性明显取决于它们的热力学性质和溶解度。因此,这项工作为液相中类似的卤化氢去除提供了指导。












































 京公网安备 11010802027423号
京公网安备 11010802027423号