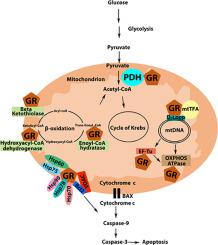
糖皮质激素是类固醇激素,可调节过多的生物作用,例如生长和代谢、免疫反应和细胞凋亡。糖皮质激素的作用是通过主要作为转录因子起作用的糖皮质激素受体介导的,但也发现它位于线粒体中。受体的线粒体定位表明受体的新功能。线粒体糖皮质激素受体 (mtGR) 相互作用蛋白的表征将阐明这些作用以及作为线粒体糖皮质激素受体输入和功能基础的生化机制。在这项研究中,应用免疫沉淀,对 HepG2 细胞和过表达线粒体靶向 GR 的 HepG2 细胞的总提取物或线粒体提取物中的 GR 相互作用蛋白进行质谱和蛋白质印迹分析,我们发现丙酮酸脱氢酶 (PDH)、伴侣蛋白如和热休克蛋白 (HSP) -60, - 70、-75 和 -90 和 78 kDa 葡萄糖调节蛋白、线粒体转录因子和酶,作为糖皮质激素受体相互作用蛋白参与调节线粒体蛋白生物合成、脂质代谢、ATP 产生和细胞凋亡。我们的研究结果揭示了该受体潜在的新型线粒体伴侣,表明 mtGR 在控制细胞线粒体相关功能方面可能具有新的调节作用。和 78 kDa 葡萄糖调节蛋白、线粒体转录因子和酶,它们作为糖皮质激素受体相互作用蛋白参与调节线粒体蛋白生物合成、脂质代谢、ATP 产生和细胞凋亡。我们的研究结果揭示了该受体潜在的新型线粒体伴侣,表明 mtGR 在控制细胞线粒体相关功能方面可能具有新的调节作用。和 78 kDa 葡萄糖调节蛋白、线粒体转录因子和酶,作为糖皮质激素受体相互作用蛋白参与调节线粒体蛋白生物合成、脂质代谢、ATP 产生和细胞凋亡。我们的研究结果揭示了该受体潜在的新型线粒体伴侣,表明 mtGR 在控制细胞线粒体相关功能方面可能具有新的调节作用。
意义
在这项研究中,线粒体 GR 相互作用蛋白的特征突出了受体在线粒体中的新调节作用。在几乎所有进行的分析中检测到 mtGR/PDH 和 mtGR/HSP60 相互作用,发现 PDH 和 HSP60 蛋白作为有效的 mtGR 结合伙伴。PDH/mtGR 相互作用的有趣发现可能表明 mtGR 参与调节糖酵解和氧化磷酸化能量产生之间的平衡。线粒体热休克-60、-70、-75 和 78 蛋白作为 mtGR 结合伙伴的表征有助于表征受体线粒体输入的生化机制。此外,鉴定线粒体热休克蛋白、代谢酶、转录因子、OXPHOS、线粒体蛋白质生物合成中的调节分子作为 mtGR 结合伙伴表明 mtGR 在糖皮质激素诱导的核和线粒体功能的调节和协调中可能具有新的调节作用,其确切的生化机制仍有待确定。该研究揭示了该受体在线粒体中潜在的新调节作用,指出了其作为控制细胞线粒体相关病理生理学的有希望的靶分子的重要性。
 "点击查看英文标题和摘要"
"点击查看英文标题和摘要"
Proteomic analysis of the mitochondrial glucocorticoid receptor interacting proteins reveals pyruvate dehydrogenase and mitochondrial 60 kDa heat shock protein as potent binding partners
Glucocorticoids are steroid hormones that regulate plethora biological actions such as growth and metabolism, immune response, and apoptosis. Glucocorticoids actions are mediated via glucocorticoid receptors which act mainly as transcription factors, but it is also found to be localized in mitochondria. Mitochondrial localization of the receptor indicates novel functions of the receptor. Characterization of the mitochondrial glucocorticoid receptor (mtGR) interacting proteins will shed light on these actions and the biochemical mechanisms that underlie mitochondrial glucocorticoid receptor import and functions. In this study, applying immunoprecipitation, mass spectrometry and Western blot analysis of the GR interacting proteins in total or mitochondrial extracts of HepG2 cells and of HepG2 cells overexpressing a mitochondrial targeted GR we found pyruvate dehydrogenase (PDH), chaperones such as and heat shock protein (HSP) −60, −70, −75 and −90, and 78 kDa glucose-regulated protein, mitochondrial transcription factors and enzymes involved in the regulation of the mitochondrial protein biosynthesis, lipid metabolism, ATP production and apoptosis as glucocorticoid receptor interacting proteins. Our results uncover potential novel mitochondrial partners of the receptor, suggesting possible new regulatory roles of mtGR in the control of mitochondrial-associated functions of the cell.
Significance
In this study the mitochondrial GR interacting proteins were characterized highlighting novel regulatory roles of the receptor in mitochondria. Detection of the mtGR/PDH and mtGR/HSP60 interaction in almost all the analyses performed uncovered PDH and HSP60 proteins as potent mtGR binding partners. The interesting finding of the PDH/mtGR interaction possibly indicates involvement of mtGR in the regulation of the balance between glycolytic and oxidative phosphorylation energy production. Characterization of the mitochondrial heat shock −60, −70, −75 and 78 proteins as mtGR binding partners contribute to the characterization of the biochemical mechanisms of the mitochondrial import of the receptor. Moreover, identification of mitochondrial heat shock proteins, metabolic enzymes, transcription factors, OXPHOS, and regulatory molecules in mitochondrial protein biosynthesis as mtGR binding partners indicates possible new regulatory roles of mtGR in the glucocorticoids-induced regulation and orchestration of nuclear and mitochondrial functions, the exact biochemical mechanism of which remain to be established. The study discloses potential new regulatory roles of the receptor in mitochondria, pointing out its importance as a promising target molecule for the control of the mitochondria-associated pathophysiology of the cell.


































 京公网安备 11010802027423号
京公网安备 11010802027423号