Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2022-02-04 , DOI: 10.1016/j.cej.2022.135029
Wenhang Wang 1, 2 , Zhengguang Ma 1 , Xiang Fei 1 , Xiaoshan Wang 1 , Zhongxue Yang 1 , Yang Wang 1, 2 , Jinqiang Zhang 1, 3 , Hui Ning 1 , Noritatsu Tsubaki 2 , Mingbo Wu 1
|
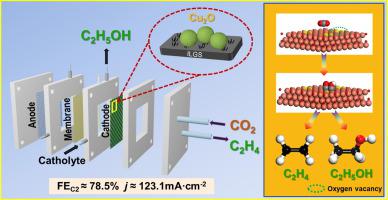
The electrochemical CO2 reduction to multi-carbon products is a promising way for tackling carbon emissions and restoring renewable electricity, which still lacks of efficient electrocatalysts. Herein, Cu2O nanoparticles with rough surface and abundant oxygen vacancies were facilely prepared by using an ionic liquid, [Omim]Cl (1-octyl-3-methylimidazolium chloride), as a bifunctional structure-directing agent, where the [Omim]+ played a role of surfactant to adjust the morphology of Cu2O and the Cl− facilitated the formation of oxygen vacancies by coordination with Cu+. The obtained Cu2O nanoparticles were further dispersed on the home-made graphite nanosheets to fabricate a composite catalyst, which showed an excellent catalytic performance with high faradaic efficiency of C2 (78.5 ± 2%) and commercial-level current density (123.1 mA cm−2) at − 1.1 V vs. RHE for 100 h in a flow cell. In situ surface-enhanced Raman spectroscopy and density functional theory calculations proved the special structure of Cu2O strengthened the adsorption of intermediates (CO2•−, CO*) and the following C–C coupling reaction, thus remarkably promoting the formation of C2 products. This work affords a novel strategy to synthesize metal oxide with controllable morphology and defects for electrochemical applications.
中文翻译:

通过离子液体联合调节 Cu2O 的形态和氧空位,可将 CO2 高效还原为 C2 产物
将CO 2电化学还原为多碳产物是解决碳排放和恢复可再生电力的一种有前途的方法,但仍缺乏高效的电催化剂。在此,通过使用离子液体[Omim]Cl(1-辛基-3-甲基咪唑氯化物)作为双功能结构导向剂,容易制备具有粗糙表面和丰富氧空位的Cu 2 O纳米颗粒,其中[Omim] +作为表面活性剂调节Cu 2 O 的形貌,Cl -通过与Cu +的配位促进氧空位的形成。得到的Cu 2将O纳米粒子进一步分散在自制的石墨纳米片上以制备复合催化剂,该催化剂表现出优异的催化性能,具有较高的C 2法拉第效率(78.5±2%)和商业级电流密度(123.1 mA cm -2)在 - 1.1 V与RHE 下,在流通池中 100 小时。原位表面增强拉曼光谱和密度泛函理论计算证明,Cu 2 O 的特殊结构加强了中间体(CO 2 •-、CO*)的吸附以及随后的C-C偶联反应,从而显着促进了C的形成2产品。这项工作为合成具有可控形态和缺陷的金属氧化物提供了一种新的策略,用于电化学应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号